Oligonucleotide, virus vector, application of oligonucleotide or virus vector, and RNAi medicinal preparation
A technology of oligonucleotides and viral vectors, applied in the medical field, can solve problems such as corneal dystrophy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Luciferase reporter system screening for efficient RNAi drugs
[0058] 1. Mammalian cell (adherent) culture
[0060] 1) Prepare warm water at 37°C-38°C in advance, take out the cells to be resuscitated from the liquid nitrogen tank, fix them with ophthalmic surgical tweezers, and quickly place them in the water to ensure that the cryopreservation tubes are completely submerged in the water to allow them to be evenly heated until frozen. The cells in the storage tube are completely thawed;
[0061] 2) Sterilize the cryotube with alcohol;
[0062] 3) Use a pipette to draw 5 mL of cell culture-based T25 cell culture flask in advance, and then use a new pipette to transfer the melted cells into the cell flask and blow it gently;
[0063] 4) Close the cap of the cell bottle, place the cell bottle in the cell incubator, 37°C, 5% CO 2 Static cultivation;
[0064] 5) After about 6-8 hours (depending on different cells), replace the fresh...
Embodiment 2
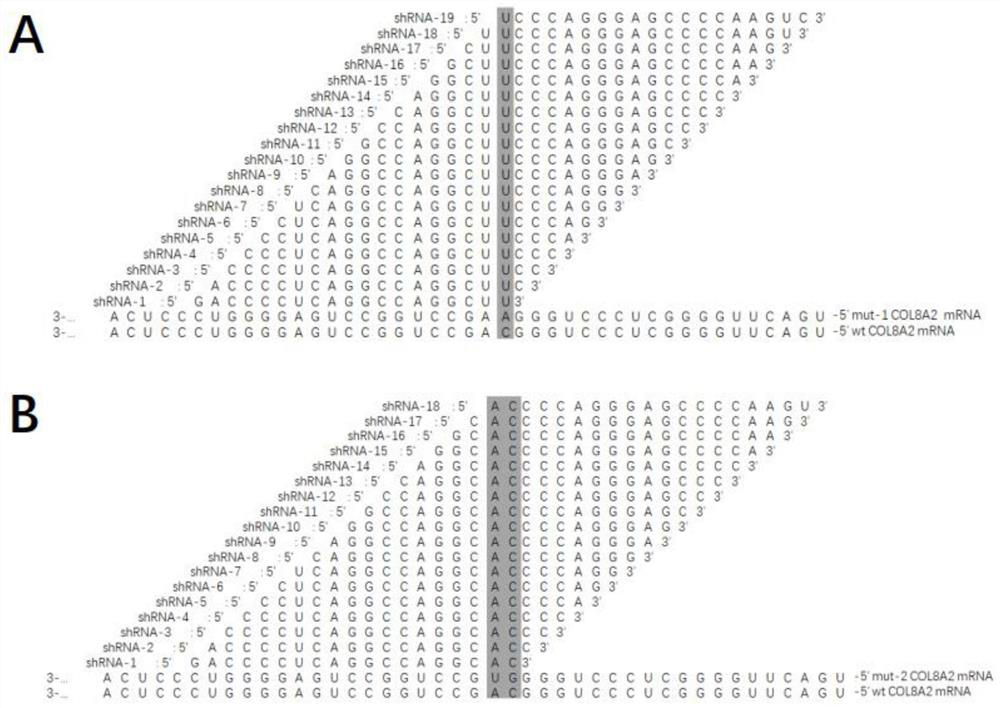
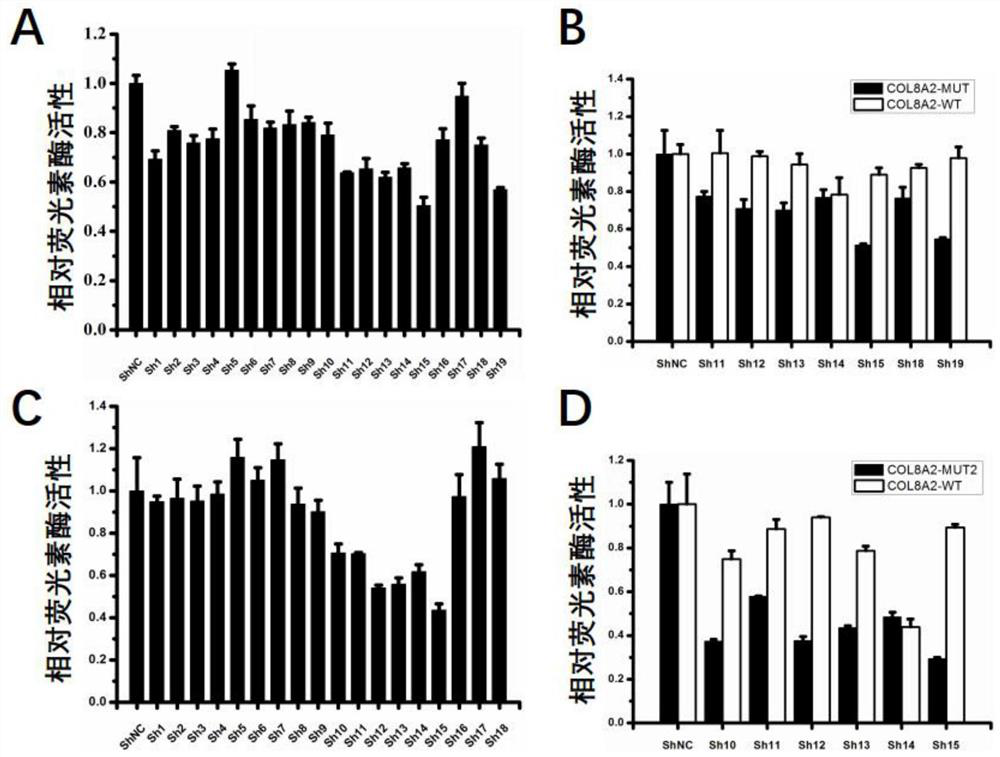
[0131] Example 2 RNAi Drug Treatment Inhibits Mutation-Specific COL8A2 Gene Expression
[0132] 1. 293 cell transfection:
[0133] The method is the same as mentioned above.
[0134] 2. Detection of COL8A2 RNA level by reverse transcription fluorescent quantitative PCR
[0135] 1. The reverse transcription reaction system is as follows:
[0136]
[0137] Reverse transcription reaction conditions: 37°C for 1h, 75°C for 10min.
[0138] 2. Real-time response system
[0139] 1) Detection primers and internal reference primers for target genes
[0140] COL8A2:5'-TCCGGCAGCCGCGAG-3'(sense)
[0141] 5'-GCATTTCCAGGTACTGGCCT-3'(antisense)
[0142] GAPDH:5'-GGAAGGTGAAGGTCGGAGTCAACGG-3'(sense)
[0143] 5'-CTCGCTCCTGGAAGATGGTGATGGG-3'(antisense)
[0144] 2) Reaction system
[0145]
[0146] 3) Reaction procedure:
[0147]
[0148] 3. AAV infection of 293 cells
[0149] 1. Prepare AAV RNAi control virus and RNAi drug virus.
[0150] 2. The MOI of the recombinant virus w...
Embodiment 3
[0171] Example 3 RNAi drugs can treat and prevent corneal dystrophy in humanized mutant COL8A2 mice
[0172] 1. AAV-RNAi virus infection and analysis of mice
[0173] 1. Construct humanized COL8A2 mutant transgenic mice.
[0174] 2. Prepare 5*10E12 vg / mL AAV RNAi control virus and RNAi drug virus.
[0175] 3. Inject 1 μL / eye of the control RNAi virus or the RNAi virus of the drug group into the eyes of 1-month-old or 6-month-old mice through anterior chamber injection.
[0176] 4. When the mice were 12 months old, the mice were killed, and the corneal tissues of the mice were separated, and stained to detect the number of corneal endothelial cells and the total protein content of COL8A2.
[0177] 2. Test results
[0178] The 1-month-old mice have not yet developed the disease, and the morphology of corneal tissue cells is normal. The 6-month-old mice have already seen corneal descemet's descemet's vegetation and loss of endothelial cells. In this example, 5*10E9 vg / eye of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com