Drug carrier, micelle, drug as well as preparation methods and application thereof
A carrier and drug technology, applied in the fields of drug carriers, micelles, medicaments and their preparation, can solve problems such as difficult to effectively control postoperative metastasis and recurrence process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
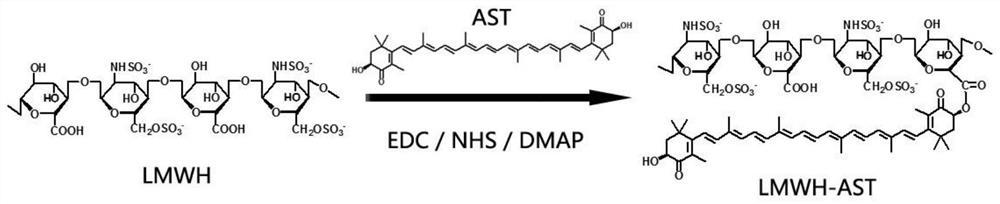
[0091] Synthesis of embodiment 1 low molecular weight heparin and hydrophobic carotenoid copolymer
[0092] The purpose of this example is to exemplarily illustrate the synthesis method of low molecular weight heparin and hydrophobic carotenoid copolymer, and the characterization of low molecular weight heparin and hydrophobic carotenoid copolymer.
[0093] 1. Preparation of enoxaparin sodium and astaxanthin copolymer:
[0094] Accurately weigh 25.0 mg of astaxanthin, and dissolve it in 24.0 mL of N,N-dimethylformamide (DMF) at room temperature. In addition, 50.0 mg of low molecular weight heparin (enoxaparin sodium) was accurately weighed, dissolved in 3.0 mL of formamide under the condition of heating in a water bath at 60°C, and then 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride (EDC) 60.0 mg, N-hydroxysuccinimide (NHS) 36.0 mg and 4-dimethylaminopyridine (DMAP) 10.0 mg were stirred and activated at 25°C for 3 hours in the dark. After the activation was c...
Embodiment 2
[0099] Example 2 Structural Characterization of Low Molecular Weight Heparin and Hydrophobic Carotenoid Copolymer (Taking Example 1 Copolymer as an Example)
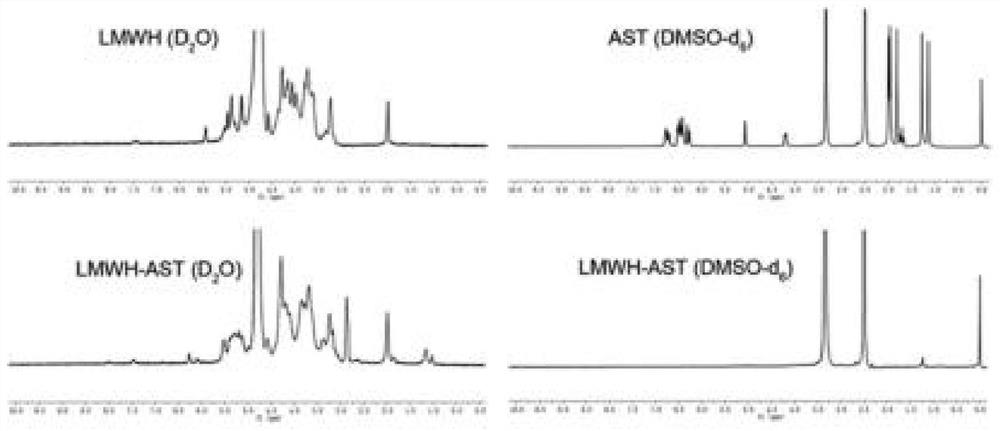
[0100] 1. The hydrogen spectrum confirmation of LMWH-AST copolymer:
[0101] Take 6.0 mg of the freeze-dried LMWH-AST sample powder, accurately weigh it, and dissolve it in 0.5 mL of D2O and DMSO-d6 respectively. And weigh 3.0mg of LMWH and 3.0mg of AST, respectively dissolved in 0.5mL of D2O and 0.5mL of DMSO-d6 as a reference sample. Proton nuclear magnetic resonance (1H-NMR) analysis was performed at 400 MHz.
[0102] The result is as figure 2 As shown, compared with the LMWH and AST spectra, the LMWH-AST sample forms forward micelles in D2O. In the spectra, a series of peaks between δ=3.5-5.5ppm are hydrogen peaks on the carbon chain skeleton in LMWH, and δ= 6.5ppm is the hydrogen peak on the carbon chain skeleton in AST. Taken together, the 1H-NMR results confirmed the successful synthesis of LANPs.
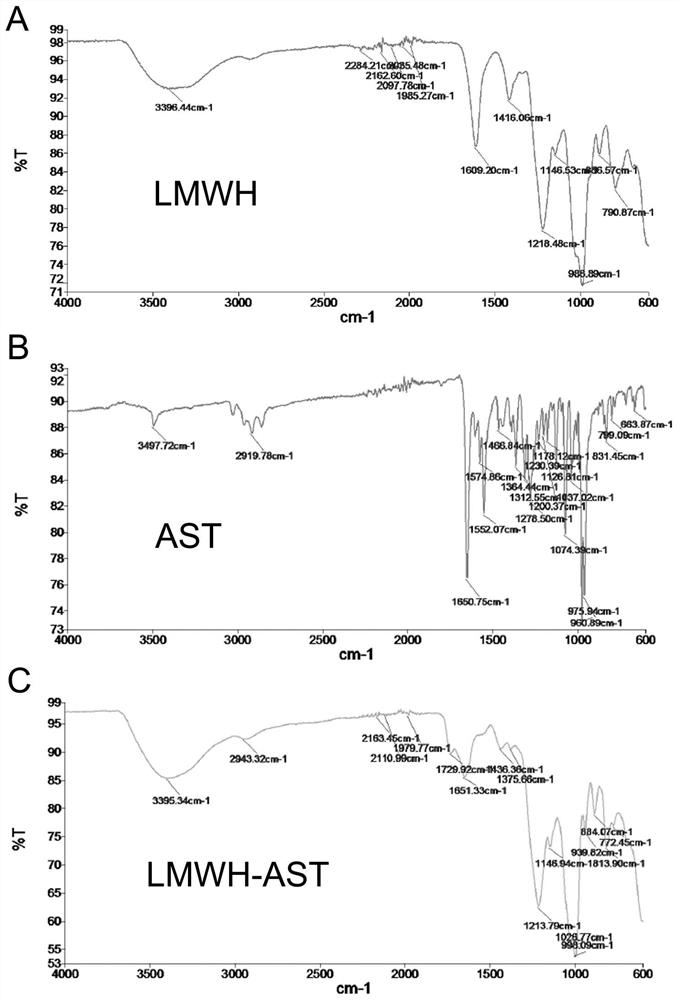
[0103] 2...
Embodiment 3
[0112] Synthesis of embodiment 3 fondaparinux sodium and astaxanthin copolymer
[0113] Accurately weigh 25.0 mg of astaxanthin, and dissolve it in 24.0 mL of N,N-dimethylformamide (DMF) at room temperature. In addition, accurately weigh 50.0 mg of fondaparinux sodium, dissolve it in 3.0 mL of formamide under the condition of heating in a water bath at 60°C, and then add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt Hydroxysuccinimide (EDC) 60.0mg, N-hydroxysuccinimide (NHS) 36.0mg and 4-dimethylaminopyridine (DMAP) 10.0mg, stirred and activated at 25°C for 3h in the dark. After the activation was completed, add 2 drops / s (about 40 μL / s) into the above-mentioned astaxanthin solution at a stirring speed of 600 rpm, and react in the dark at 30°C for 48 hours under the protection of argon (Ar). After the reaction was completed, the reaction solution was added into 3 times the volume of acetone at 0° C., shaken thoroughly, and then centrifuged at 4000 rpm at room temperature...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com