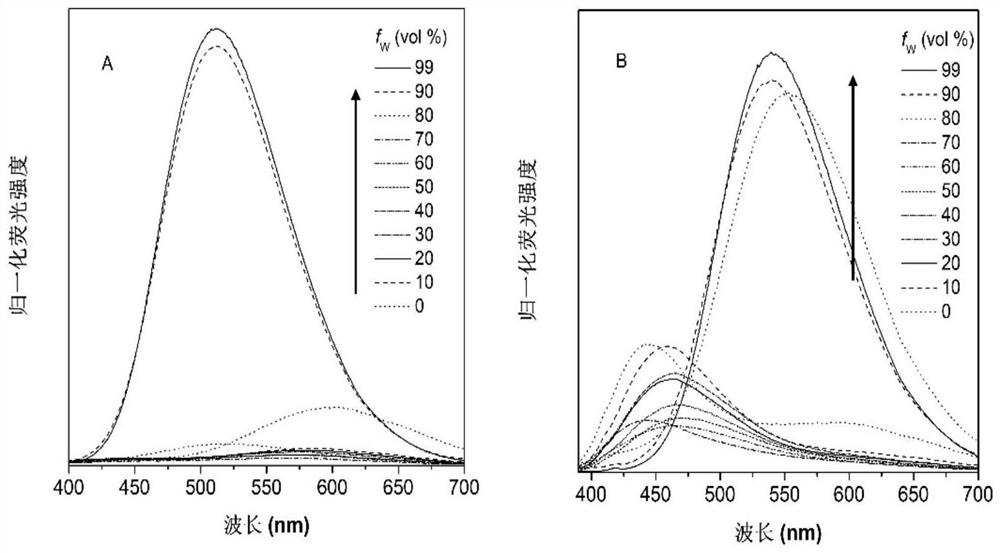
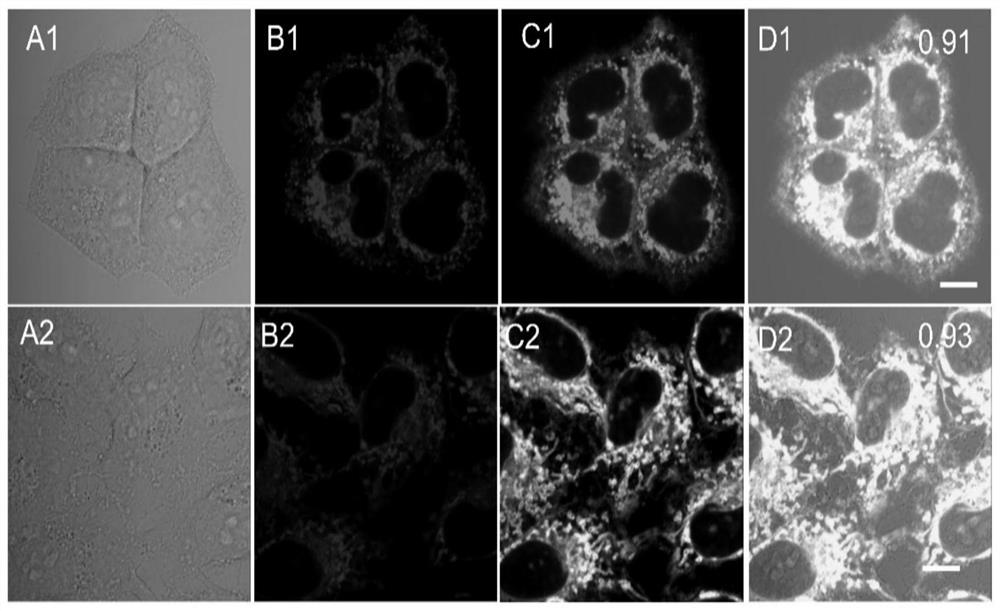
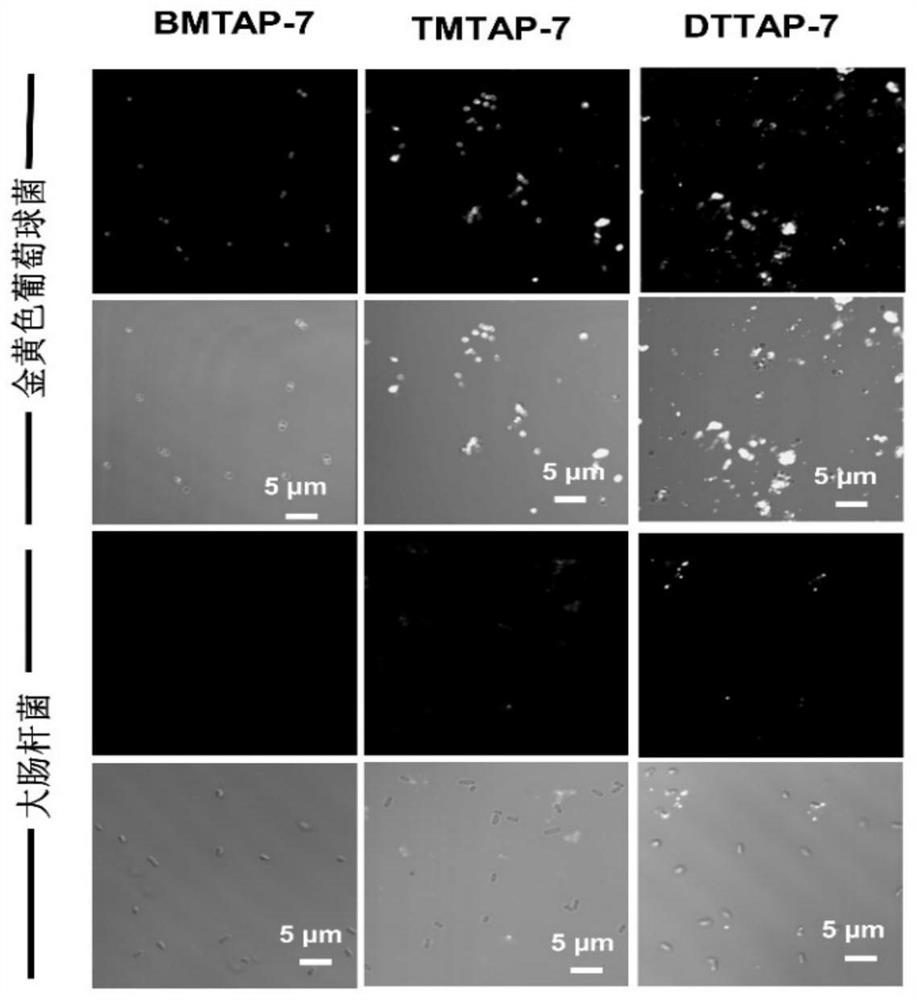
Tricyclic 2-amino pyridinium salt fluorescent probe as well as preparation method and application of fluorescent probe
An aminopyridinium salt, fluorescent probe technology, applied in the field of biomedical materials, can solve the problems of reducing sensitivity, aggregation-induced luminescence quenching, reducing signal-to-noise ratio, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1 Preparation of a fluorescent probe (BMTAPC-7)
[0079]
[0080] The synthetic route is as follows:
[0081]
[0082] 4-bromobenzoyl chloride (10mmol, 2.19g), 4-methoxyphenylacetylene (10mmol, 1.32g), PdCl 2 (PPh 3 ) 2 (0.2mmol, 144mg) and cuprous iodide (0.4mmol, 76mg) were added to a 250mL two-necked round-bottomed flask, then evacuated with a pump, replaced with nitrogen three times for 3 times; then added dry dichloromethane (100mL) . Triethylamine (11 mmol, 1.5 mL); the reaction was stirred at room temperature for 5 h (reaction progress was monitored by TLC). After the substrate reaction is complete, slowly add anhydrous AlCl to the reaction system 3 (1mmol, 133mg) and DBU (30mmol, 4.5mL), the reaction mixed solution was stirred at room temperature for 2h (TLC monitoring until the reaction was complete), the mixed reaction solution was filtered to remove the catalyst, the filtrate was concentrated, acidified with 20mL of 1M hydrochloric acid, ...
Embodiment 2
[0084] Embodiment 2 Preparation of a fluorescent probe (BMTAPC-5)
[0085]
[0086] The synthetic route is as follows:
[0087]
[0088] 4-bromobenzoyl chloride (10mmol, 2.19g), 4-methoxyphenylacetylene (10mmol, 1.32g), PdCl 2 (PPh 3 ) 2 (0.2mmol, 144mg) and cuprous iodide (0.4mmol, 76mg) were added to a 250mL two-necked round-bottomed flask, then evacuated with a pump, replaced with nitrogen three times for 3 times; then added dry dichloromethane (100mL) , triethylamine (11mmol, 1.5mL), the reaction was stirred at room temperature for 5h (reaction progress was monitored by TLC), and after the substrate had completely reacted, anhydrous AlCl was slowly added to the reaction system 3 (1 mmol, 133 mg) and DBN (30 mmol, 4.5 mL). The reaction mixed solution was stirred at room temperature for 2 h (TLC monitoring until the reaction was complete), the mixed reaction solution was filtered to remove the catalyst, the filtrate was concentrated, acidified with 20 mL of 1M hydroc...
Embodiment 3
[0090] Embodiment 3 Preparation of a fluorescent probe (BMTAP-7)
[0091]
[0092] The synthetic route is as follows:
[0093]
[0094] The solid BMTAPC-7 (1mmol, 485mg) synthesized in Example 1 was dissolved in acetone, an aqueous solution of potassium hexafluorophosphate (2mmol, 368mg) was added, stirred for 2h, the mixture was added with water and then filtered, and the filtered solid was washed with water to remove inorganic salts , dried to give 535 mg of yellow solid BMTAP-7 in 90% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com