A class of drug derivatives of moxifloxacin and their preparation method and application
A technology of moxifloxacin and derivatives, which is applied in the field of biomedical materials, can solve the problems that the fluorescence properties of drugs are rarely studied, and the application of fluorescence properties and drug activity is few, achieving excellent inhibitory effect, good selectivity, and reaction site point clear effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] A kind of preparation of moxifloxacin drug derivative (MXF-P)
[0061] The synthetic route is as follows:
[0062]
[0063] MXF-P: Compound 1 (304 mg, 0.6 mmol) and MXF-HCl (219 mg, 0.5 mmol) were dissolved in a mixed solution of MeCN:DMF=9mL:1mL, and KHCO was added 3 (84 mg, 1.0 mmol); the reaction mixture was heated to 90° C. and reacted for 12 hours. After the solvent was removed by rotary evaporation under reduced pressure, water was added to the mixture. It was then extracted with DCM (3 x 30ml); the organic layers were combined and washed with anhydrous Na 2 SO 4 Drying, concentration, and silica gel column purification (eluent DCM:MeOH=20:1) gave MXF-P as a yellow solid in 58% yield. 1 H NMR (500MHz, CDCl 3 )δ15.16(brs,1H),8.37(s,1H),7.84–7.75(m,9H),7.71–7.65(m,7H),4.02–3.99(m,1H),3.80–3.71(m, 3H), 3.65–3.55 (m, 5H), 3.17 (brs, 1H), 2.76 (brs, 1H), 2.53 (brs, 1H), 2.39–2.23 (m, 3H), 1.82–1.41 (m, 10H) ,1.37–1.17(m,5H),0.99–0.92(m,2H). 13 C NMR (125MHz, ...
Embodiment 2
[0065] Preparation of Anions in the Structure of Moxifloxacin Derivatives
[0066] will get Br - The compound MXF-P (0.2 mmol, 166 mg) was dissolved in acetone, and an aqueous solution of potassium hexafluorophosphate (0.3 mmol, 55 mg) was added and stirred for 2 h. Water was added to the mixture and filtered. The filtered solid is washed with water to remove inorganic salts, and dried to obtain a product containing hexafluorophosphate anion.
Embodiment 3
[0068] Absorption and Fluorescence Characterization of Moxifloxacin Derivatives
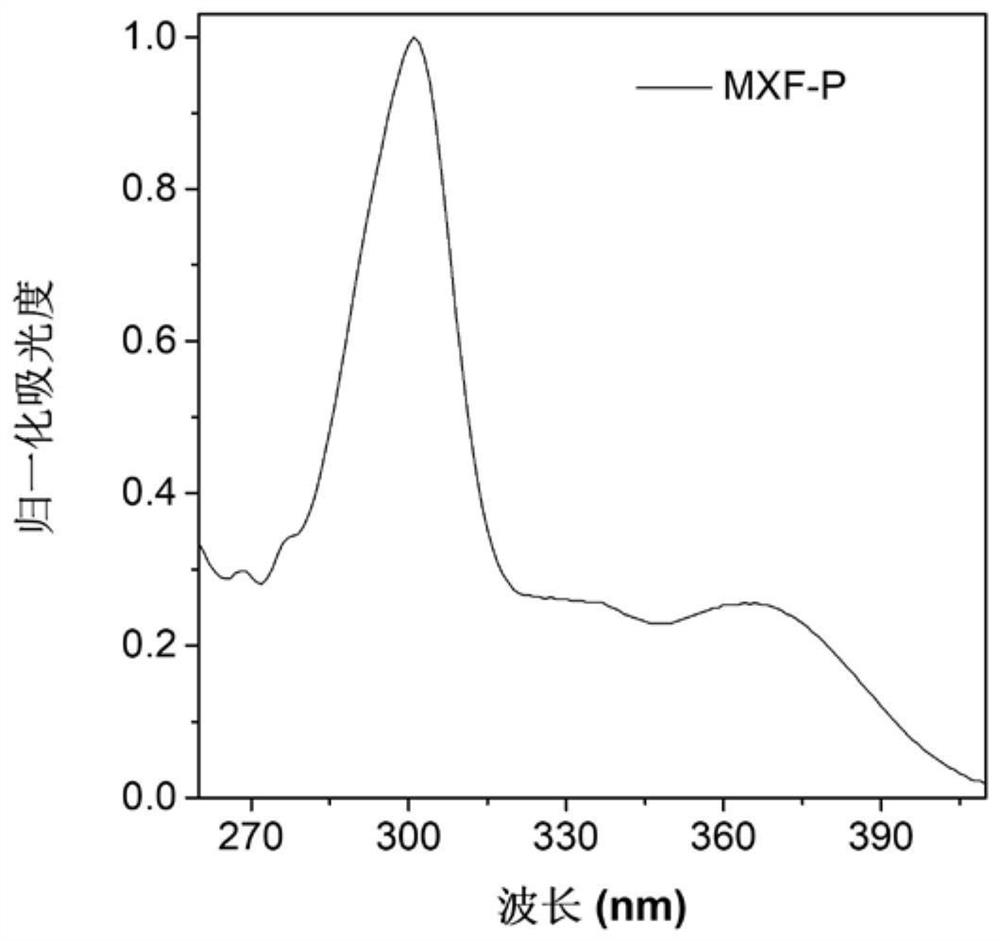
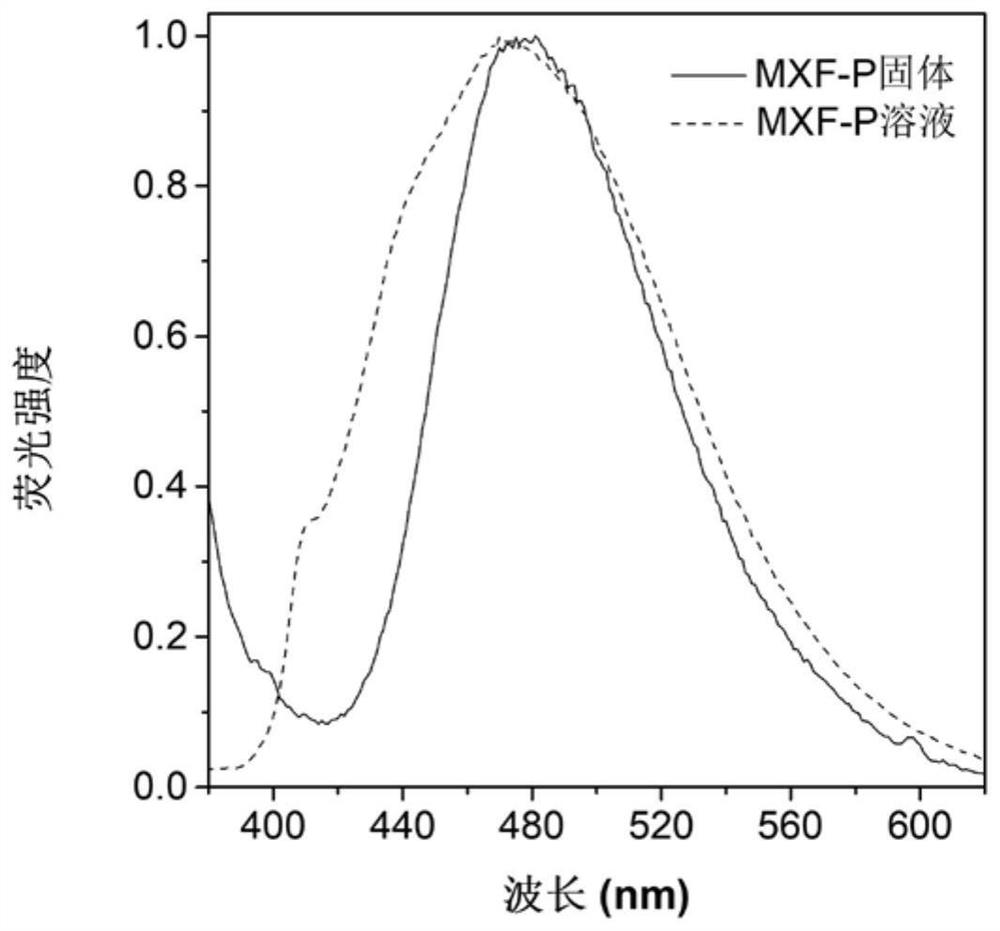
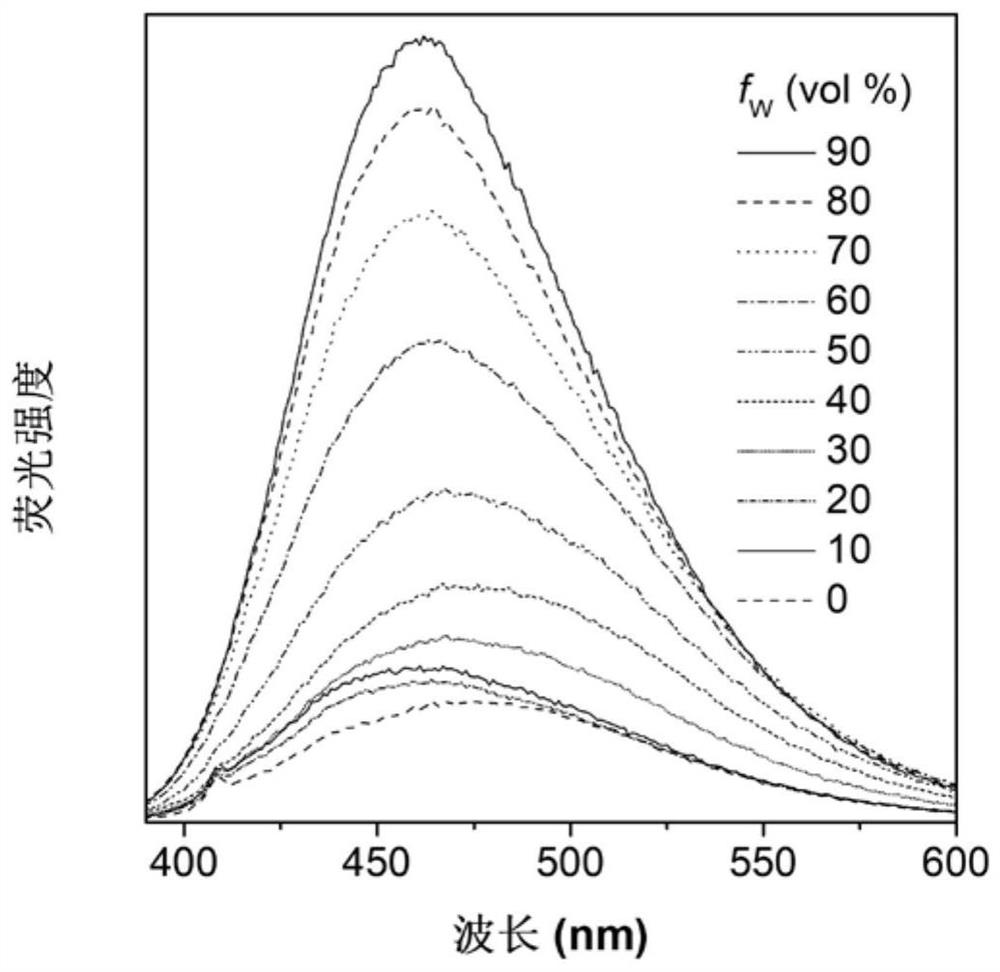
[0069] Figure 1A Based on the absorption spectrum of the material MXF-P obtained in Example 1 in DMSO, it can be seen that the main absorption peak of the molecule is around 365 nm; Figure 1B is the fluorescence emission spectrum of MXF-P in DMSO solvent and solid state, it can be seen that the emission peak of this molecule is mainly around 475nm; Figure 1C for with H 2 O content increased in MXF-P (10 μM) in DMSO / H 2 Fluorescence emission spectrum in O(v / v) mixed solvent; when the molecule is in DMSO solution, the luminescence is weak, with the addition of water, it can be found that the fluorescence intensity is continuously enhanced, when the water content reaches 90%, The fluorescence intensity reaches a maximum value. This phenomenon is due to the continuous enhancement of fluorescence due to the aggregation of MXF-P molecules as the proportion of poor solvent water increases. These ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com