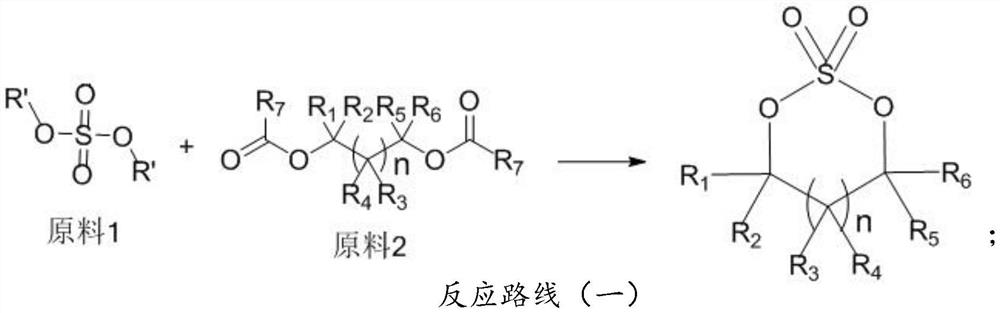
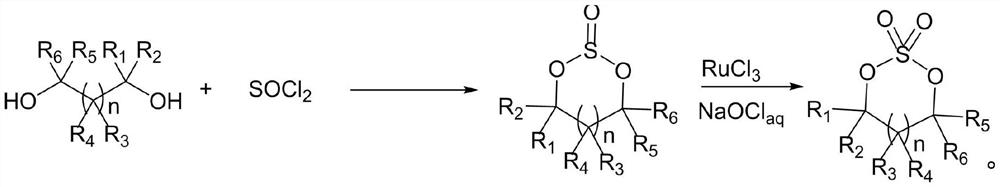
Preparation method of cyclic sulfate
A technology of cyclic sulfate and dihydrocarbyl sulfate is applied in the field of preparation of cyclic sulfate, and can solve the problems of difficult control of reaction heat release, equipment corrosion, large salt content and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
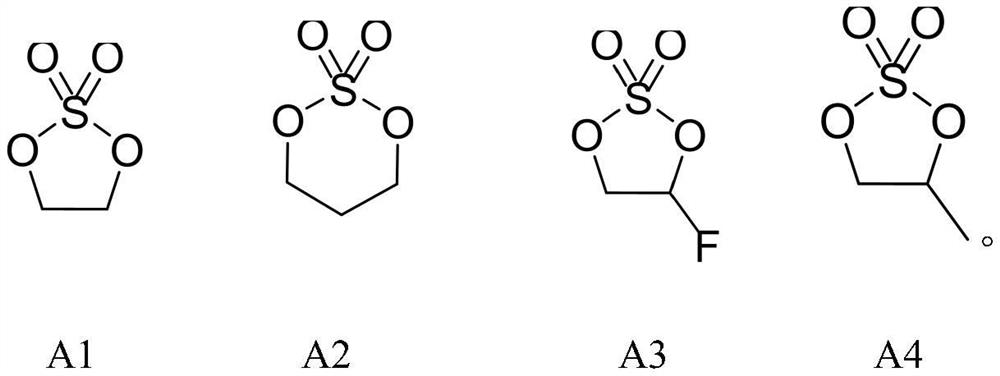
[0028] The preparation of compound A1 vinyl sulfate, concrete steps are as follows:
[0029] A 1000mL three-necked flask was equipped with a vacuum distillation device, and 146.1g (1.0mol) of ethylene glycol diacetate, 151.6g (1.2mol) of dimethyl sulfate and 75g of activated clay were added to a 1000mL three-necked flask, and magnetically stirred. N 2 (50mL / min) replacement, control the vacuum of the reaction system to 1800-2000Pa, raise the temperature to 120°C, the system boils slightly, the vacuum distillation receiving bottle gradually distills out fractions, and the obtained fractions are methyl acetate, which can be sold or recycled ;
[0030] Control the internal temperature at 120-125°C and keep it warm until no fraction distills out, stop the reaction, lower the temperature to 20-30°C, add 500g of dichloroethane, stir for 30min, filter with suction, filter out activated clay, and desolventize the obtained filtrate to Slight solid precipitation in the system;
[003...
Embodiment 2
[0034] The preparation of compound A2 propylene sulfate, concrete steps are as follows:
[0035] 1000mL three-necked flask, equipped with a vacuum distillation device, weighed 102.09g (1.0mol) propylene carbonate, 151.6g (1.2mol) dimethyl sulfate and 63g activated clay into the 1000mL three-necked flask, magnetically stirred, N 2 (50mL / min) displacement, control the vacuum of the reaction system to 1500-1700Pa, raise the temperature to an internal temperature of 125°C, the system boils slightly, and a fraction is gradually distilled out from the receiving bottle of vacuum distillation, and the obtained fraction is dimethyl carbonate, which can be sold or recycled use;
[0036] Control the internal temperature at 125-130°C and keep it warm until no fraction distills out, stop the reaction, lower the temperature to 20-30°C, add 600g of dichloromethane, stir for 30min, filter with suction, filter out activated clay, and desolventize the obtained filtrate to the system Slight sol...
Embodiment 3
[0040] The preparation of compound A3 fluoroethylene sulfate, concrete steps are as follows:
[0041] A 1000mL three-necked flask, equipped with a vacuum distillation device, weighed 106.0g (1.0mol) of fluoroethylene carbonate, 138.7g (1.1mol) of dimethyl sulfate and 48g of activated clay into a 1000mL three-necked flask, magnetically stirred, N 2 (50mL / min) replacement, control the vacuum of the reaction system to 1500-1700Pa, raise the temperature to 105°C, the system boils slightly, the vacuum distillation receiving bottle gradually distills out fractions, and the obtained fractions are dimethyl carbonate, which can be sold or recycled use;
[0042] Control the internal temperature at 105-110°C and keep it warm until no fraction distills out, then stop the reaction. Cool down to 20-30°C, add 500g of dichloromethane, stir for 30 minutes, filter with suction, filter out activated clay, and desolventize the obtained filtrate under reduced pressure until the system has a sligh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com