Nitrogen oxide removal catalyst and preparation method thereof
A technology of nitrogen oxides and catalysts, which is applied in the field of nitrogen oxides removal catalysts, can solve the problems of poor low-temperature activity and easy sulfur poisoning, and achieve the effects of reducing adsorption, reducing the formation of ammonium sulfate salts, and promoting decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031]In order to better realize the above technical solution, the present invention also provides a method for preparing the nitrogen oxide removal catalyst based on cobalt, lanthanum and niobium oxide nanocages, comprising the following steps:
[0032] Step S1. Preparation of cobalt, lanthanum and niobium oxide nanocages, including:
[0033] Step S11. Prepare methanol solutions of cobalt nitrate and 2-methylimidazole respectively, add the 2-methylimidazole solution into the methanol solution of cobalt nitrate under vigorous stirring, stir well and let stand at room temperature for 24-48h, separate, Wash with ethanol and dry at 50-80°C for 2-4 hours to obtain a solid, and then disperse the solid in the ethanol solution;
[0034] Step S12. Add lanthanum acetate and niobium acetate to the mixed solution of ethanol and water, then add the mixed solution of lanthanum acetate and niobium acetate to the solution in step S11 under stirring, and let stand at room temperature after fu...
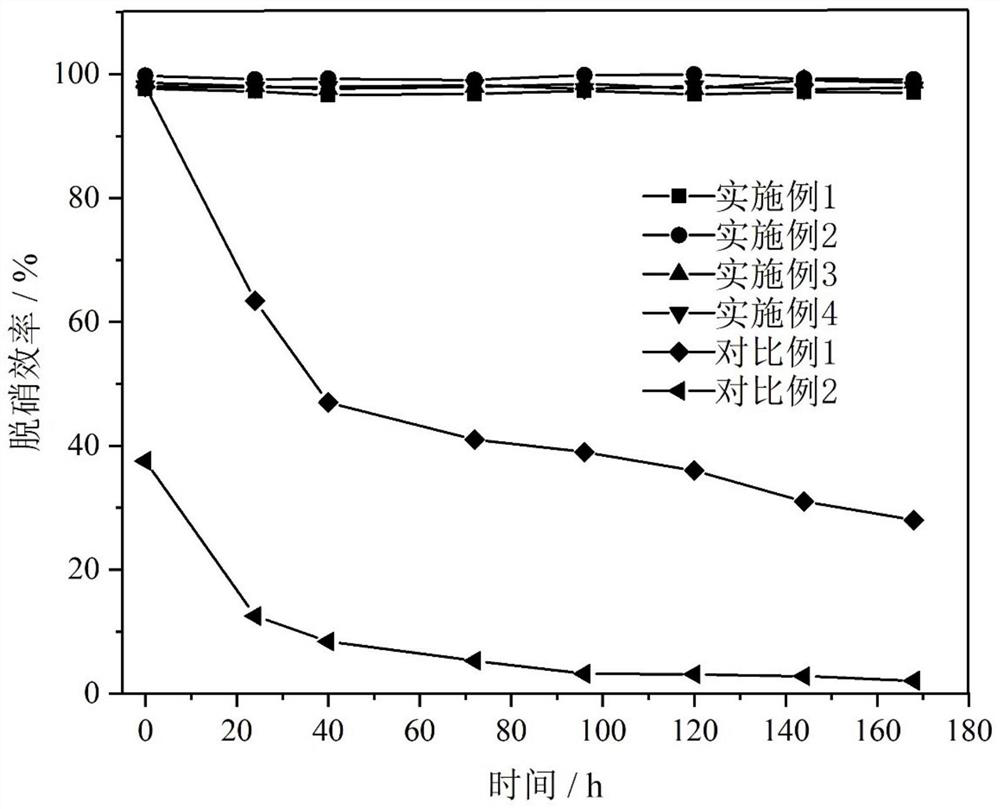
Embodiment 1
[0047] A nitrogen oxide removal catalyst based on cobalt, lanthanum and niobium oxide nanocages and a preparation method thereof. The specific steps of the preparation method include:
[0048] (1) Preparation of cobalt, lanthanum and niobium oxide nanocages
[0049] Weigh 193.26g of cobalt nitrate hexahydrate and 54.51g of 2-methylimidazole and dissolve them in 1.328L and 166mL of methanol respectively, add the 2-methylimidazole solution into the methanol solution of cobalt nitrate under vigorous stirring, and stir at room temperature Stand still for 24 hours, separate, wash with ethanol, and dry at 50°C for 2 hours to obtain a solid, and then disperse the solid in the ethanol solution;
[0050] Add 104.93g of lanthanum acetate and 128.82g of niobium acetate into a mixed solution of 664mL of ethanol and water, then add the mixed solution of lanthanum acetate and niobium acetate into the cobalt solution under stirring, and let it stand at room temperature for 24 hours after ful...
Embodiment 2
[0057] Another nitrogen oxide removal catalyst based on cobalt, lanthanum and niobium oxide nanocages and a preparation method thereof. The specific steps of the preparation method include:
[0058] (1) Preparation of cobalt, lanthanum and niobium oxide nanocages
[0059] Weigh 115.26g of cobalt nitrate hexahydrate and 32.51g of 2-methylimidazole and dissolve them in 792mL and 99mL of methanol respectively, add the 2-methylimidazole solution into the methanol solution of cobalt nitrate under vigorous stirring, and after stirring fully Stand still for 48 hours, separate, wash with ethanol, and dry at 80°C for 4 hours to obtain a solid, and then disperse the solid in an ethanol solution;
[0060] Add 125.15g of lanthanum acetate and 153.65g of niobium acetate into a mixed solution of 396mL of ethanol and water, then add the mixed solution of lanthanum acetate and niobium acetate into the cobalt solution under stirring, and let it stand at room temperature for 48h after fully sti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com