Composition and preparation method thereof
A technology of composition and medicine, which is applied in the field of immunity-enhancing composition and its preparation, can solve problems such as low human immunity, and achieve the effect of safe and effective consumption, good health care effect, and no incompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
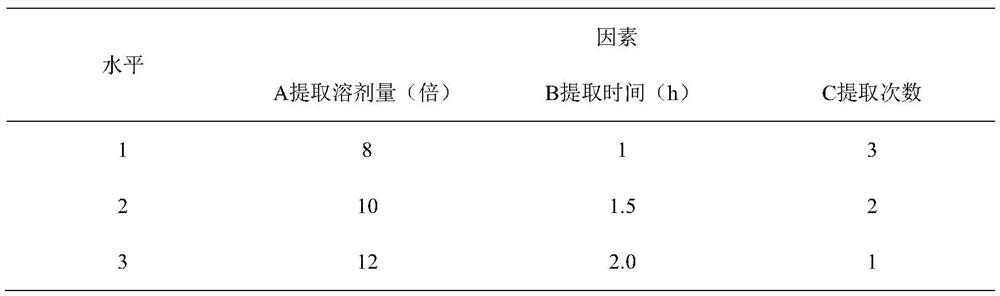
[0041] 1. Selection of extraction process
[0042] Take 80g of Cistanche medicinal material, 80g of Astragalus medicinal material, 80g of medlar medicinal material, and 80g of Ligustrum lucidum medicinal material in each part, take 9 parts in total, extract according to the relevant parameter requirements of factor level table 1, and measure echinacoside and verbascoside, astragalus in the extract glycoside transfer rate and crude polysaccharide content. Taking echinacoside, verbascoside, transfer rate of astragaloside IV and crude polysaccharide content as the investigation index, L 9 (3 4 ) Orthogonal test to investigate the amount of extraction solvent, extraction time, and extraction times.
[0043] Table 1 Factor levels
[0044]
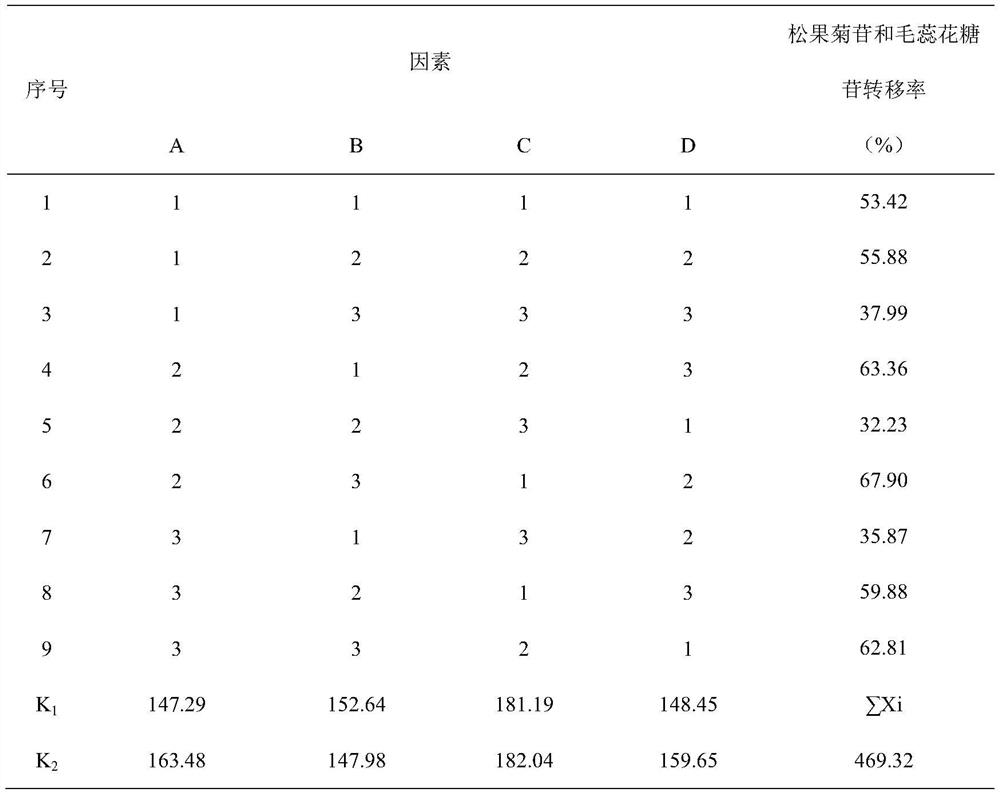
[0045] Table 2L 9 (3 4 ) Orthogonal table (echinacoside, verbascoside)
[0046]
[0047]
[0048] Table 3 variance analysis table (echinacoside, verbascoside)
[0049]
[0050] Table 4 L 9 (3 4 ) Orthogonal Table (Astragalos...
Embodiment 2
[0067] 1. Extraction, concentration and drying process of medicinal materials
[0068] Weigh 3.0 kg of Cistanche deserticola, 3.0 kg of Astragalus membranaceus, 3.0 kg of Ligustrum lucidum and 3.0 kg of Lycium barbarum according to the proportion, add 1200 L of water, and reflux extraction twice, each time for 1.0 h. After filtration, the filtrate was concentrated under reduced pressure at 60°C to obtain a concentrated thick paste with a relative density of 1.25. Continue drying under reduced pressure at 70°C, crush, pass through a 65-mesh sieve, and make dry cream powder, the weight of which is 7.95kg.
[0069] 2. Selection of types and dosage of excipients
[0070] 2.1 Selection of types of excipients
[0071] After experimental research, it is necessary to add appropriate excipients to overcome problems such as caking, poor fluidity, large difference in grain weight, and difficulty in disintegration during the preparation process. In the selection process of the diluent,...
Embodiment 3
[0097] Weigh 54kg of Cistanche deserticola, 54kg of Astragalus membranaceus, 54kg of Ligustrum lucidum and 54kg of Lycium barbarum according to the proportion, add 2160L of water, and reflux extraction twice, each time for 1.0h. After filtering, the filtrate was concentrated under reduced pressure at 70°C to obtain a concentrated thick paste with a relative density of 1.20. Continue to dry under reduced pressure at 70°C for 5 hours, pulverize, pass through a 65-mesh sieve, and make a dry cream powder. The weight of the dry cream powder is 47.73kg, and then add auxiliary materials in proportion to the dry cream powder, and add microcrystalline cellulose 9.82kg, add 5.04kg of cornstarch, mix well, moisten with 90% ethanol, pass through a 20 mesh sieve, granulate, dry at 60°C for 5 hours, pass through a 20 mesh sieve, granulate, add disintegrant cross-linked carboxymethyl to the granules Sodium cellulose 1.92kg and lubricant 1.30kg are mixed to obtain about 63.24kg of particle we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com