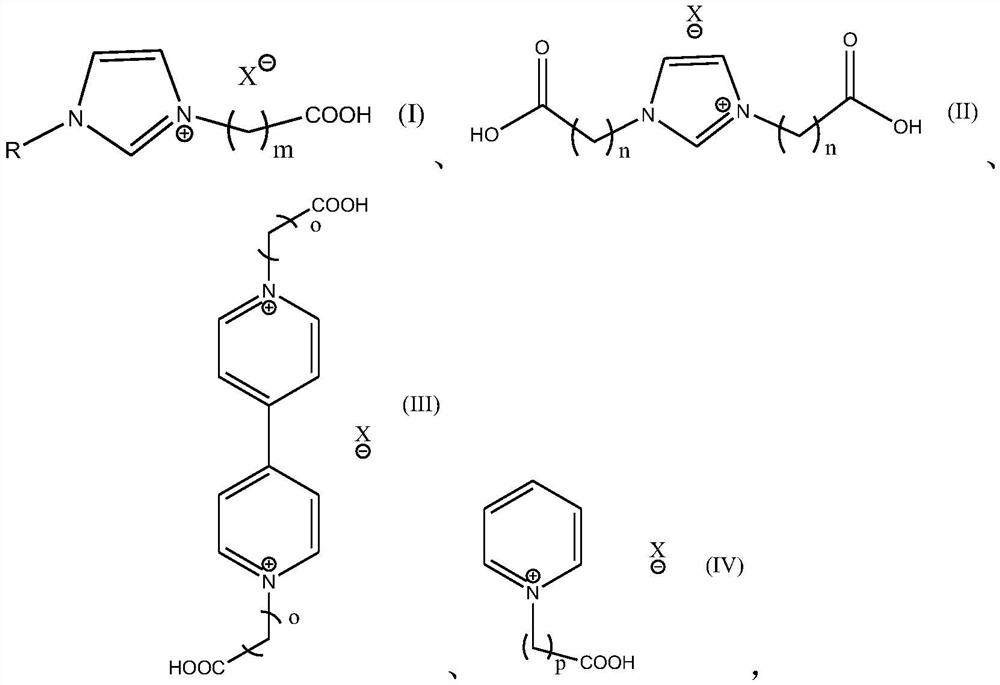
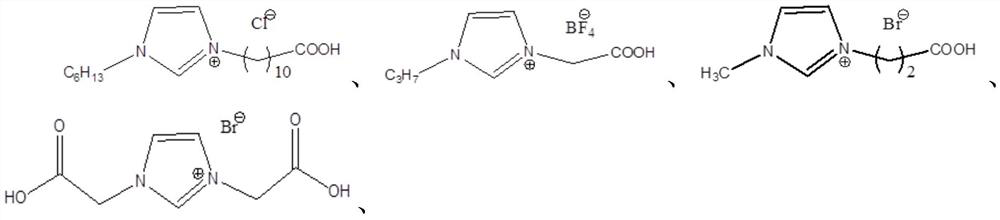
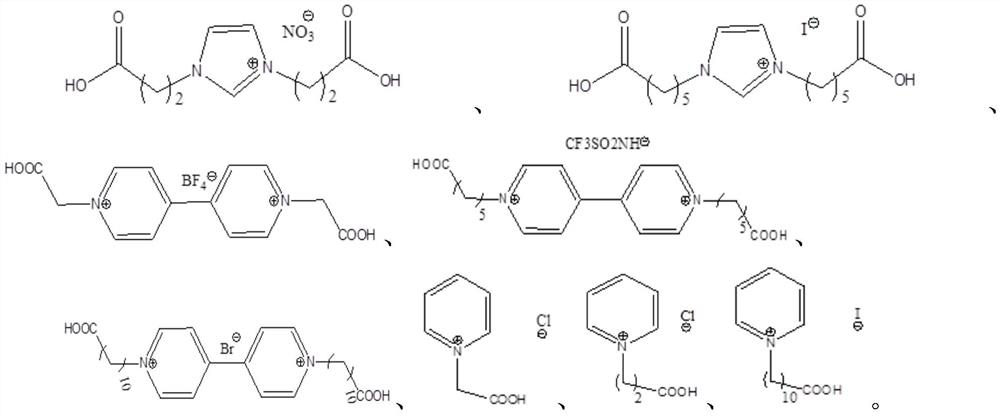
A kind of method for preparing 3-hydroxypropionaldehyde by hydration of acrolein
A technology for hydration of acrolein and hydroxypropionaldehyde, which is applied to the preparation of carbon-based compounds, preparation of organic compounds, chemical instruments and methods, etc., can solve problems such as unstable operation of solid catalysts, achieve small investment, stable process, and high selectivity Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1-12N
[0045] Preparation Example 1-12N heterocyclic carboxylic acid ionic liquid catalyst
[0046] 1. Preparation of Example 1-3 carboxyalkyl-alkylimidazole ionic liquids: add 0.05mol haloalkane acids to the round bottom flask respectively (Example 1-3 is respectively chloroacetic acid, 10-bromodecanoic acid, 3- bromopropionic acid), then mixed with 10mL of acetone, shaken until completely dissolved, and then added an equimolar amount of N-alkylimidazole (N-propylimidazole, N-hexylimidazole, N-methylimidazole respectively in Examples 1-3 imidazole), at 80°C, after reacting for 12h, the imidazole carboxylic acid ionic liquid IL-1, IL-2 and IL-3 with the anion being a halogen were obtained, and IL-3 was the carboxane used in Example 3 base-alkylimidazole ionic liquid catalyst; IL-1 and IL-2 were ion-exchanged with equimolar 40% tetrafluoroboric acid and 36wt% hydrochloric acid respectively, and continued to react for 60 minutes. After the reaction was completed, suction filtration, T...
preparation Embodiment 7-9
[0048] 3. Preparation Example 7-9N,N-dicarboxyalkyl-4,4'-bipyridine ionic liquid: 4,4'-bipyridine (1.2mmol) and haloalkylcarboxylic acid at a molar ratio of 1.2:1 (Embodiments 7-9 are respectively bromoacetic acid, 6-bromohexanoic acid, and 10-bromodecanoic acid) as raw materials, respectively dissolved in 20mL CH 2 Cl 2 In a round-bottomed flask, the oil bath was heated to reflux at 60°C and stirred for 24 hours, and a pale yellow precipitate was formed. Cool to room temperature and wash with 3 x 5ml CH 2 Cl 2 Vacuum drying after washing and filtering to obtain ionic liquids IL-7, IL-8 and IL-9, IL-9 is the target ionic liquid in Example 9, IL-7 and IL-8 were mixed with tetrafluoroboric acid and Example 7-8 N,N-dicarboxyalkyl-4,4'-bipyridine ionic liquid can be obtained by ion exchange with lithium salt of trifluoromethanesulfonimide.
[0049] 4. Preparation of Example 10-12 N-carboxyalkylpyridine ionic liquid: pyridine (0.11mol) and haloalkyl carboxylic acid (respectivel...
Embodiment 1-12
[0051] Add deionized water, acrolein, and N-heterocyclic carboxylic acid ionic liquid catalysts in a certain proportion into a 1L reactor, and continue to stir for a certain period of time to obtain a hydration reaction solution, which is sampled and analyzed to calculate the conversion rate and selectivity;
[0052] The hydration reaction solution is extracted by sugar-ionic liquid two-phase aqueous phase: the sugar solution with a mass concentration of 20-50% is added dropwise to the acrolein hydration reaction solution, and the hydration reaction solution is placed in a water bath at 25-45°C. Stop oscillating until turbidity appears (this point is the cloud point of the system), continue to drop the sugar solution, and let it stand for 30-60min to separate layers, that is, the two-phase aqueous system. The upper water phase is an ionic liquid-rich water phase, composed of ionic liquid, 3-HPA, and a small amount of water, acrolein, acrolein dimer and other by-products; the lo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com