Vaccine universal vectors and preparation method and application thereof
A vaccine carrier and vaccine technology, applied in the field of vaccine universal carrier protein and its preparation, can solve the problems of weak particle uniformity, time-consuming, manpower and material resources, and affecting immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1HB
[0123] Example 1HBc-S and HBc (1-183) Preparation of -S vaccine carrier protein
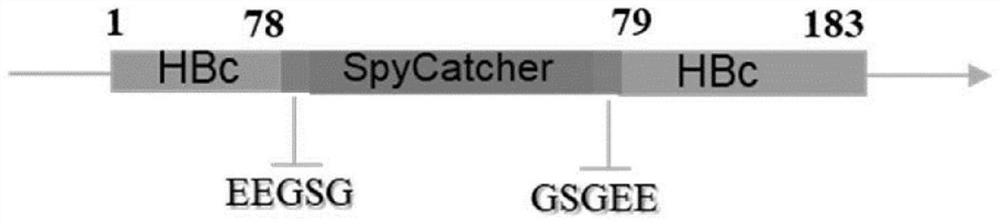
[0124] The amino-terminal truncated ΔN1SpyCatcher gene fusion was inserted between the 78th and 79th amino acids of the main immunodominant region of the truncated HBc (1-149), constructed as follows Figure 1A The shown vaccine carrier protein HBc-S; the coding gene fusion of amino terminal truncated ΔN1SpyCatcher is inserted between the 78th and 79th amino acids of the main immunodominant region of full-length HBc (1-183), constructed as Figure 1B The indicated vaccine carrier protein HBc (1-183) -S; In order to ensure the correct self-assembly of HBc and the correct display of SpyCatcher on the surface of HBc VLPs, introduce GSG linker and two glutamic acid (E) on both sides of the insertion site of ΔN1SpyCatcher gene, HBc-S-pBR and HBc (1-183) -S-pBR was constructed by Sangon Bioengineering (Shanghai).
[0125] HBc-S-pBR or HBc (1-183) -S-pBR was transformed into BL21 competent cells, 3 t...
Embodiment 2
[0127] Example 2 Application of HBc-S to prepare chimeric virus-like particle vaccines displaying linear, circular and modified epitopes
[0128] (1) Assembly characteristics of HBc-S
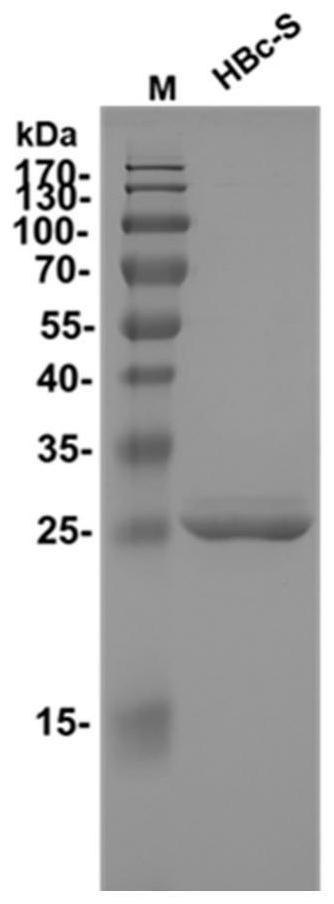
[0129] Concentrate the purified HBc-S to 3 mg / mL, and wash with 20 mM PBS (pH=5), 20 mM PBS (pH=6.2), 20 mM PBS (pH=8) and citric acid reaction buffer (Citrate Buffer, pH=6.2 ) diluted to 0.2mg / mL, add Protein gel stain (250×diluted); truncated HBc (1-149) was used as a control, heated from 20°C to 95°C using a real-time fluorescent quantitative PCR instrument, and the heating rate was 1°C / min.
[0130] The result is as Figure 4A , Figure 4B , Figure 4C , Figure 4D with Figure 4E As shown, the truncated HBc VLPs only had a Tm value of 83°C, indicating the existence of the VLP structure; while HBc-S only showed a Tm value of 83°C in the citric acid reaction buffer, indicating that HBc-S was in the citric acid solution Stable assembly into VLPs.
[0131] (2) Preparation of HBc-S-P V...
Embodiment 3
[0142] Example 3 Application of HBc-S-pTau422 Vaccine to Treat Tau.P301S Transgenic Mice
[0143] (1) Preparation of HBc-S-pTau422 vaccine
[0144]Mix HBc-S VLPs and SpTau422 at a molar ratio of 1:3, add 3 times the volume of citrate binding reaction buffer, and leave it at room temperature for 1-3 hours; then the mixture is ultrafiltered with a 100kDa cut-off membrane, Unreacted peptide SpTau422 was removed (citric acid reaction buffer as ultrafiltration liquid).
[0145] Figure 11A It is the result of SDS-PAGE detection, the electrophoretic band of HBc-S-pTau422 moves up, slightly larger than that of HBc-S, and the purity is higher, indicating that the SpTau422 polypeptide has undergone an adhesion reaction with HBc-S, realizing the full integration of HBc-S modification; Figure 11B It is the transmission electron microscope result picture, HBc-S-pTau422 maintains the intact VLP structure; Figure 11C Shown as a particle size distribution graph, the particle size of th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com