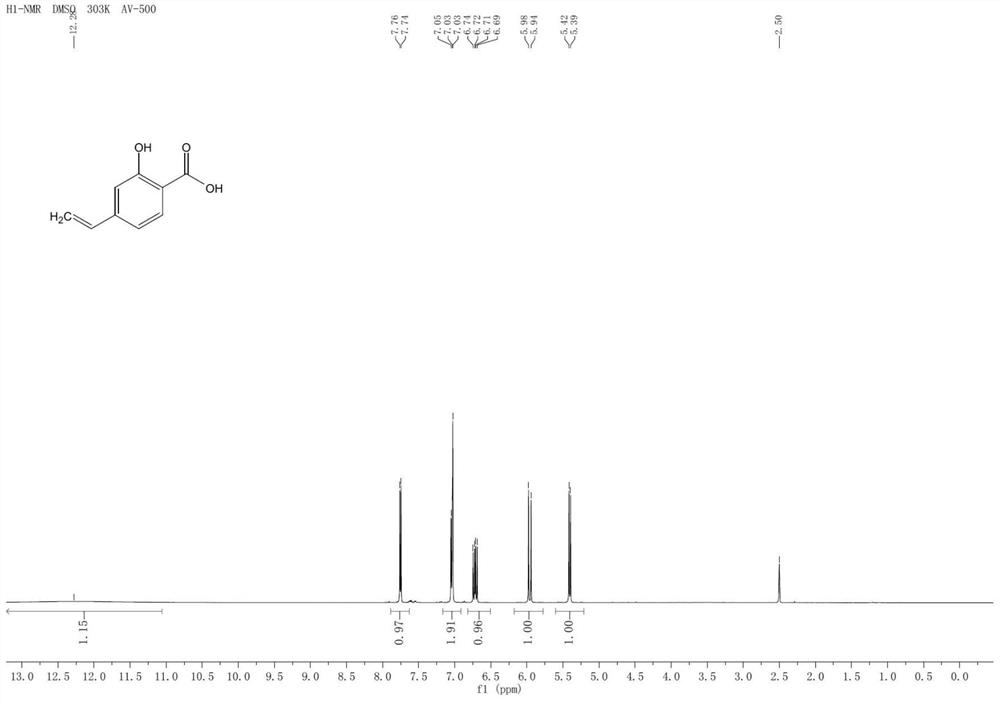
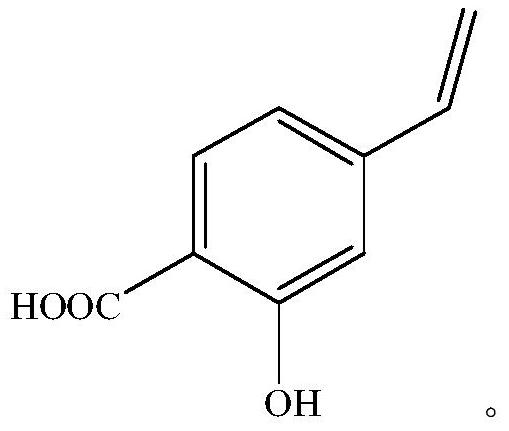
Preparation method of p-vinylsalicylic acid
A technology of vinyl salicylic acid and p-aminosalicylic acid, which is applied in the field of preparation of p-vinyl salicylic acid, can solve the problems of unfavorable large-scale application, easy to be oxidized, and high price, and achieve industrial scale The effect of production, reducing the difficulty of post-processing, and streamlining the operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Stir 5mmol (765mg) p-aminosalicylic acid and 7.5mmol (0.65mL) 36% concentrated hydrochloric acid at room temperature for 15min, cool to 0°C and slowly add 7.5mmol (518mg sodium nitrite dissolved in 2.2mL water) sodium nitrite aqueous solution dropwise Continue stirring for 15 min, then add 7.5 mmol (1.43 g) CuI in batches and continue stirring for 30 min at room temperature to generate p-iodosalicylic acid.
[0039] After the reaction, without post-treatment, the reaction solution was directly transferred to the hydrothermal reaction kettle, and 17.5mmol (700mg) NaOH, 0.05mmol (46mg) Pd were added to the reaction mixture 2 (dba) 3 , 10mmol (1.9g) of triethoxyvinylsilane and 5mL of methanol, the temperature was raised to 100°C and the reaction was continued for 10h. After cooling down, add 1M HCl to adjust the pH value to neutral, extract and separate the liquid, take the organic phase and dry it, remove the organic phase by rotary evaporation, and obtain p-vinyl salicy...
Embodiment 2
[0041] Stir 5mmol (765mg) p-aminosalicylic acid and 7.5mmol (0.65mL) 36% concentrated hydrochloric acid at room temperature for 15min, cool to 0°C and slowly add 7.5mmol (518mg sodium nitrite dissolved in 2.2mL water) sodium nitrite aqueous solution dropwise Stirring was continued for 15 min, and then 7.5 mmol (1.25 g KI was dissolved in 2.2 mL water) KI aqueous solution was slowly added dropwise and stirring was continued for 30 min at room temperature to generate p-iodosalicylic acid.
[0042] After the reaction, without post-treatment, the reaction solution was directly transferred to the hydrothermal reaction kettle, and 17.5mmol (700mg) NaOH, 0.05mmol (46mg) Pd were added to the reaction mixture 2 (dba) 3 , 10mmol (1.9g) of triethoxyvinylsilane and 5mL of methanol, the temperature was raised to 100°C and the reaction was continued for 10h. After cooling down, add 1M HCl to adjust the pH value to neutral, extract and separate the liquids, take the organic phase and dry it...
Embodiment 3
[0044] Stir 5mmol (765mg) p-aminosalicylic acid and 7.5mmol (0.65mL) 36% concentrated hydrochloric acid at room temperature for 15min, cool to 0°C and slowly add 7.5mmol (518mg sodium nitrite dissolved in 2.2mL water) sodium nitrite aqueous solution dropwise Stirring was continued for 15 min, and then 7.5 mmol (1.25 g KI was dissolved in 2.2 mL water) KI aqueous solution was slowly added dropwise and stirring was continued for 30 min at room temperature to generate p-iodosalicylic acid.
[0045] After the reaction was finished, the reaction liquid was directly transferred to the hydrothermal reaction kettle without post-treatment, and 17.5 mmol (700 mg) NaOH, 0.05 mmol (265 mg) 5% Pd / C, 10 mmol (1.9 g) Tris Ethoxyvinylsilane and 5mL of methanol were heated to 100°C to continue the reaction for 10h. After cooling down, add 1M HCl to adjust the pH value to neutral, extract and separate the liquid, take the organic phase to dry, remove the organic phase by rotary evaporation, obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com