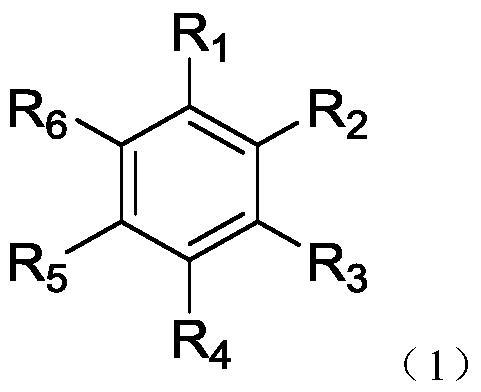
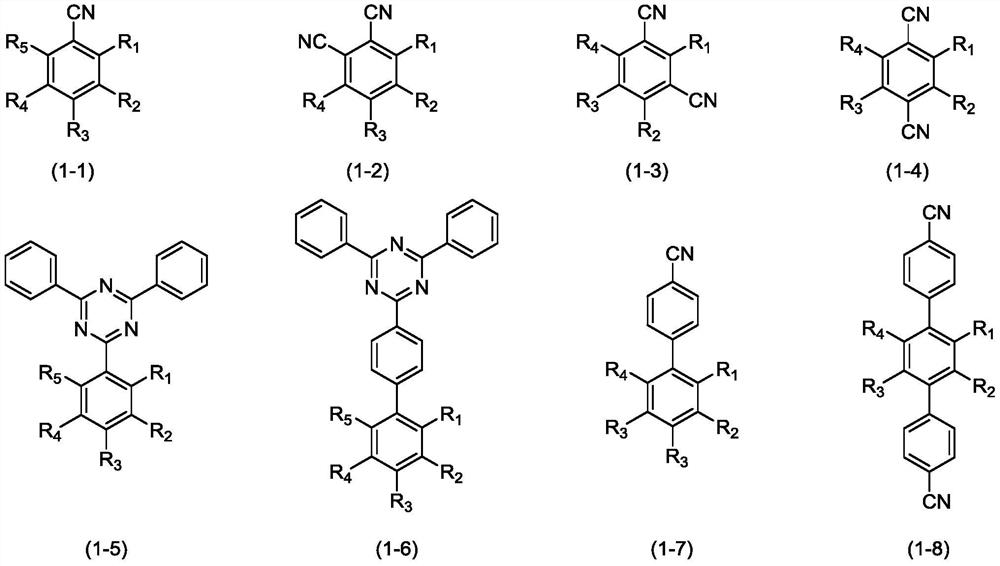
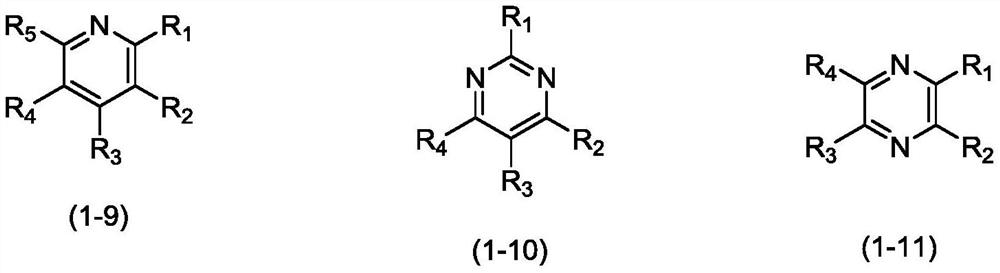
Organic compound, application thereof and organic light-emitting device adopting compound
An organic compound and heteroaryl technology, applied in the field of organic electroluminescence, to achieve the effects of high luminous efficiency, excellent service life, and low starting voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] Preparation of Intermediate 1:
[0057] In the reaction kettle, carbazole (16.7g, 0.1mol), heavy water (D 2 O, 50mL) and PtO 2 (0.23g, 1mmol), heated at 250°C for 14h, cooled and filtered to obtain 17g of white solid, yield 96%.
[0058] Preparation of compound C1:
[0059] Under a nitrogen atmosphere, in a 1000ml three-necked flask, dissolve sodium tert-butoxide (19.2g, 0.2mol) in 100mL MF and stir for 2 hours, then dissolve the DMF solution of deuterated carbazole (17g, 0.1mol) dropwise Add and stir for 1 hour after the addition is complete. Then, a DMF solution in which pentafluorobenzonitrile (3.86 g, 0.02 mol) was dissolved was added dropwise, and heated and stirred at 80° C. overnight. Subsequently, the reaction solution was poured into water, and the solid was obtained by filtration, which was separated and purified by chromatographic column to obtain a yellow solid C1 with a yield of 90%.
[0060] Product mass spectrum (m / e): 968.2, elemental analysis: theo...
Embodiment 1
[0569] The glass plate coated with the ITO transparent conductive layer is ultrasonically treated in a commercial cleaning agent, rinsed in deionized water, ultrasonically degreased in acetone: ethanol mixed solvent, baked in a clean environment until the water is completely removed, and then cleaned with ultraviolet light. Light and ozone cleaning, and bombardment of the surface with a beam of low-energy cations;
[0570] HATCN was vacuum evaporated on the ITO transparent conductive layer as the hole injection layer of the device, the evaporation rate was 0.1nm / s, and the total film thickness was 5nm;
[0571] On the hole injection layer, NPB was vacuum evaporated as the hole transport layer of the device, the evaporation rate was 0.1nm / s, and the total film thickness was 30nm;
[0572] TCAC was vacuum evaporated on the hole transport layer as the first exciton blocking layer, the evaporation rate was 0.1nm / s, and the total film thickness was 10nm;
[0573] The light-emittin...
Embodiment 2 to Embodiment 21
[0577] Example 2 to Example 21 are all prepared in the same way as Example 1, the difference is that the luminescent dye in the light-emitting layer is replaced by the compound C1 of the present invention with the compounds C2, C3, C4, C5, C6, C7, C8, C9, C10, C11, C12, C13, C14, C15, C16, C17, C33, C49, C65, C81.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com