Fluorescence and lutetium-177 double-labeled biomolecule as well as preparation method and application thereof
A biomolecular, dual-labeling technology, used in chemical instruments and methods, fluorescence/phosphorescence, material analysis by optical means, etc., can solve the problems of limited data and rare research on such biomolecules, and achieve long residence time, easy to use. Separation and purification, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
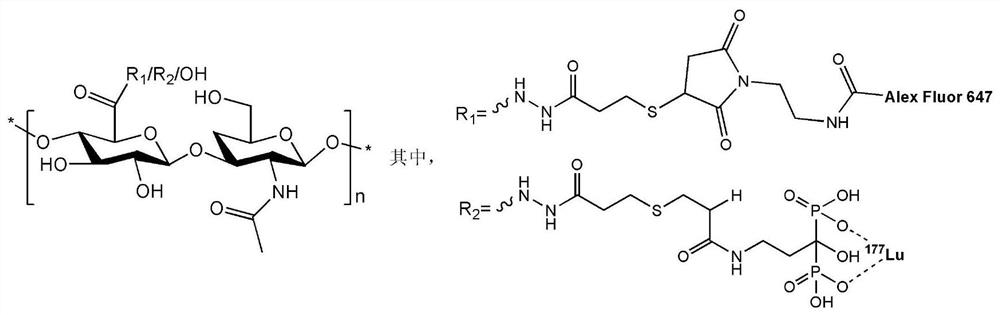
[0033] In this example, a method for preparing a hyaluronic acid polymer compound double-labeled with lutetium-177 and Alexa Fluor 647 is provided, and the steps are as follows:
[0034] (1) Dissolve 100 mg of hyaluronic acid in 12 mL of pure water, add 8.8 mg of functionalized linker small molecule 3,3'-dithiobis(propionic hydrazide) (3,3'-Dithiobis(propionic hydrazide)) and Stir and mix, then add the HoBt solution formed by dissolving 35.5mg 1-hydroxybenzotriazole (HoBt) in 0.5mL DMSO and stir and mix; adjust the pH value of the resulting mixed solution to 4.7 with 1mol / L hydrochloric acid, Then 35mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) was added, and the reaction was stirred at room temperature for 8h;
[0035] (2) Adjust the pH value of the reaction solution obtained in step (1) to 8.7 with 1mol / L sodium hydroxide, then add 25mg dithiothreitol (DTT), and adjust with 1mol / L hydrochloric acid after stirring at room temperature for 6h When the...
Embodiment 2
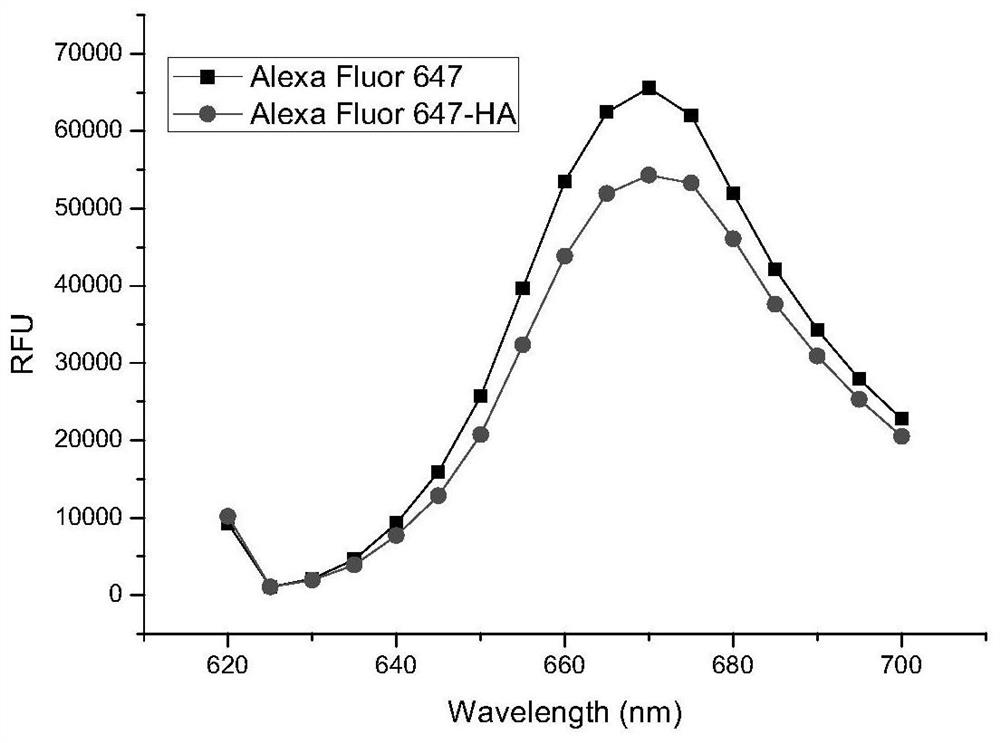
[0039] In this example, the in vitro stability of the lutetium 177 / fluorescence double-labeled hyaluronic acid compound prepared in Example 1 was investigated.
[0040] Take lutetium 177 / Alexa Fluro 647 fluorescent double-labeled hyaluronic acid macromolecular compound 0.1mL (1mg / ml) of Example 1, place them in 1mL of PBS, DMEM culture solution and serum respectively, and place them at a constant temperature of 37°C for 2h. After 8h and 24h, the solution was taken respectively to detect the label stability by iTLC. The results showed that the hyaluronic acid polymer compound with lutetium 177 / Alexa Fluro647 fluorescent double labeling had good stability within 24 hours.
Embodiment 3
[0042] In this example, the in vivo imaging of lutetium 177 / fluorescent double-labeled hyaluronic acid compound was investigated.
[0043] Inject 0.1 mL of lutetium 177 / AlexaFluro 647 fluorescent double-labeled hyaluronic acid polymer compound with a concentration of 2.5 mg / ml into small cell lung cancer tumor mice through the tail vein, and observe the fluorescent signal at 180 hours. The result shows that the fluorescent label It has good stability in vivo, and it can be observed that the targeting molecule reaches the target tumor location and stays in the tumor for a long time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com