Method for detecting genotoxic impurity haloalkane in aripiprazole
A detection method, aripiprazole technology, is applied in the field of detection of genotoxic impurity halogenated alkanes in aripiprazole, which can solve the problems of low limit, difficult analysis, no detection method of halogenated alkanes, etc., and achieve good durability , good specificity, good theoretical plate number and symmetry factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
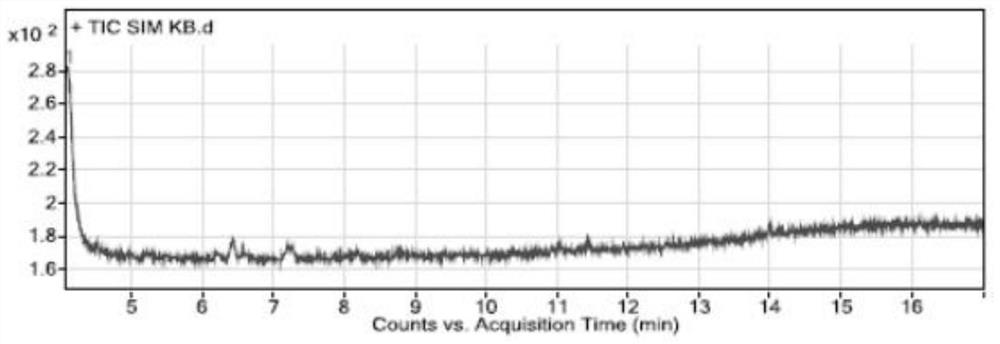
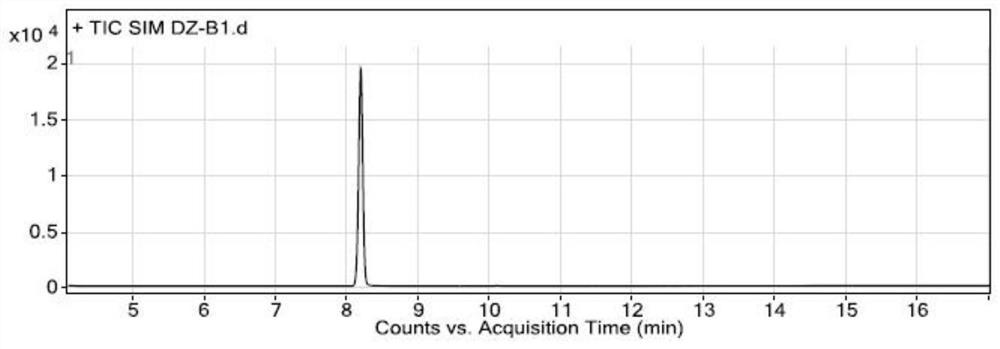
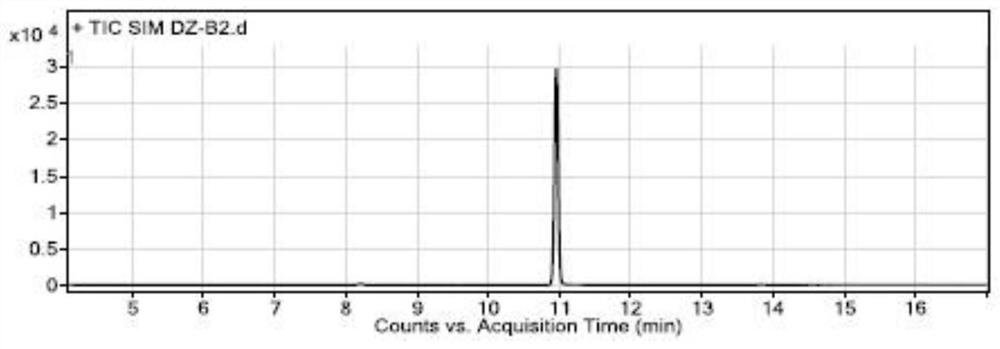
[0075] Specificity and system suitability experiment:
[0076] The conditions of gas chromatography-mass spectrometry detection in the present embodiment are:
[0077] Gas chromatography conditions: direct injection: 1 μL; chromatographic column: a capillary column with 6% cyanopropylphenyl-94% dimethyl polysiloxane (or similar in polarity)) as a stationary liquid as a chromatographic column (Agilent DB -624, 30m×0.25mm×1.4μm); carrier gas is helium; flow rate: 1.0mL / min; temperature program: the initial temperature is 100°C, keep it for 5 minutes, and raise the temperature to 150°C at a rate of 5°C per minute, and maintain 2 minutes; inlet temperature 180°C; split ratio 10:1;
[0078] Mass spectrometry conditions: transfer line temperature 200°C, ion source temperature 230°C, quadrupole temperature 150°C, ion monitoring mode (SIM), extracted ions are m / z55.00; m / z91.00; m / z135.00, stationed Dwell time 100ms, solvent delay time 4 minutes, low resolution mode.
[0079] Exclu...
Embodiment 2
[0088] Durability test:
[0089] The preparation method of the aripiprazole-halogenated alkane mixed solution is the same as that of Example 1.
[0090] The flow rate in the gas chromatographic conditions is respectively set to 0.9mL / min and 1.1mL / min, and other conditions are consistent with embodiment 1, and the aripiprazole-halogenated alkane mixed solution is carried out to gas chromatography-mass spectrometry detection, and the obtained spectrum is as follows Figure 8 ~ Figure 9 shown;
[0091] Set the initial temperature of the chromatographic column in the gas chromatography conditions to 98°C and 102°C respectively, and the other conditions are the same as in Example 1, and the aripiprazole-halogenated alkane mixture is detected by gas chromatography-mass spectrometry, and the obtained spectrum is as follows Figure 10 ~ Figure 11 shown.
[0092] The split ratios in the gas chromatographic conditions are respectively set to be 9:1 and 11:1, and other conditions are ...
Embodiment 3
[0095] Solution stability testing:
[0096] The preparation method of need testing solution and reference substance solution is identical with embodiment 1, respectively at 0h, 2h, 4h, 6h, 8h, 12h, 16h and 24h need testing solution and reference substance solution are carried out gas chromatography-mass spectrometry detection , detection condition is identical with embodiment 1, and gained result is shown in table 2:
[0097] Table 2 Aripiprazole solution and reference substance solution stability test result
[0098]
[0099] According to Table 2, it can be seen that the deviation of the peak area and 0h of the reference substance solution and the test solution within 24h is less than 20%, indicating that the two can be kept stable for 24h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com