Cell glycocalyx protection effect of anisodamine
An anisodamine, cell technology, applied in the field of medicine, health food or food
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
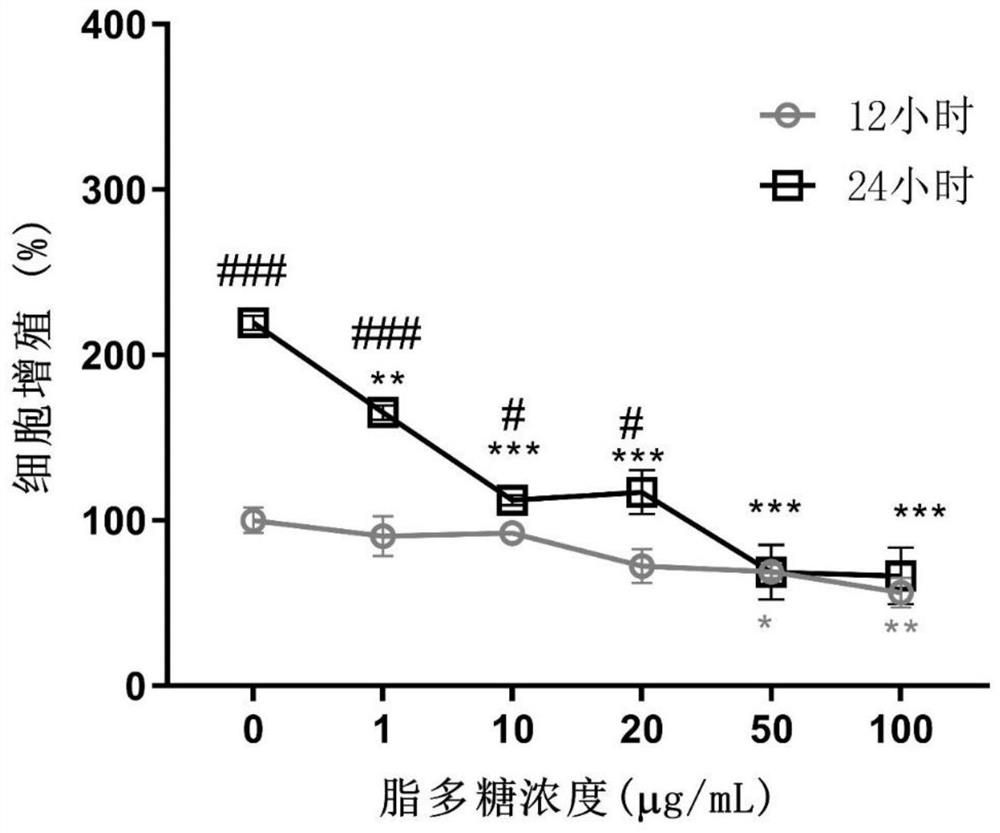
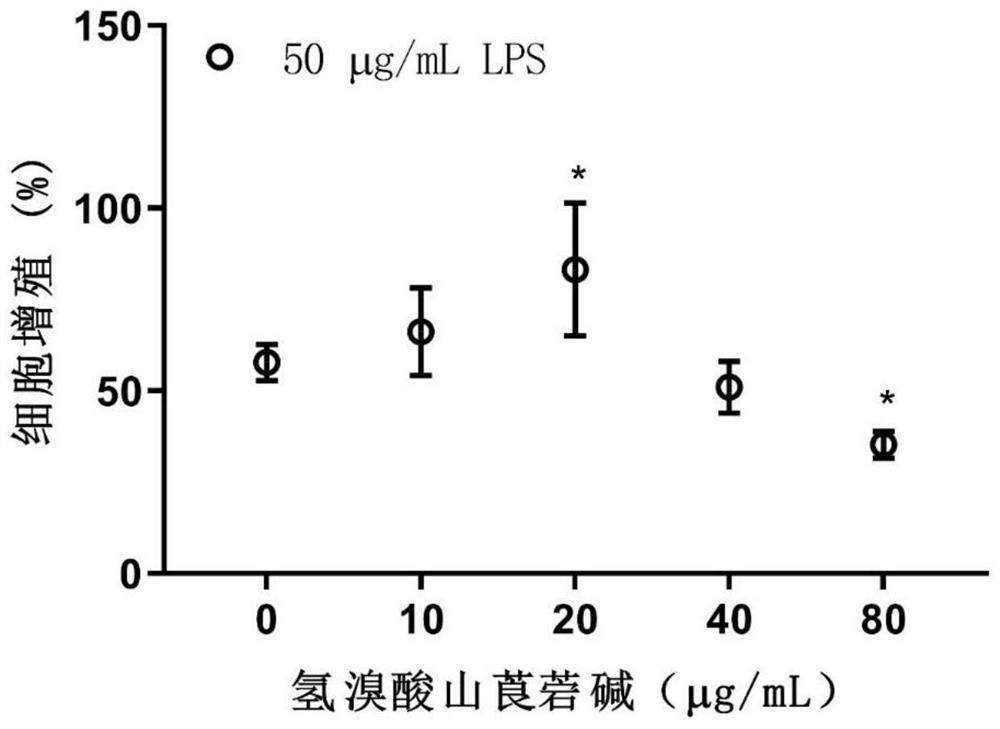
[0061] Embodiment 1: the effect of anisodamine hydrobromide on LPS-induced hCMEC / D3 cell proliferation
[0062] 1.1 Cell culture and treatment
[0063] Immortalized human brain microvascular endothelial cells hCMEC / D3 were obtained from Beijing Beinan Chuanglian Institute of Biotechnology (BNBIO), China. hCMEC / D3 endothelial cells were incubated with endothelial cell medium (ECM; cat.1001, Sciencell, USA) at 37°C and 5% CO 2 cultivated under conditions. Anisodamine hydrobromide was obtained from Chengdu First Pharmaceutical Co., Ltd., Chengdu, China, dissolved in 20 mg / mL distilled water, and filtered through a 0.22 μm membrane filter. Prepare anisodamine hydrobromide at final concentrations of 10, 20, 40, and 80 μg / mL using ECM. LPS was dissolved in distilled water at 2 mg / mL, and 1, 10, 20, 50, and 100 μg / mL of LPS were prepared with ECM. hCMEC / D3 cells were treated with LPS and anisodamine hydrobromide.
[0064] 1.2 Cell proliferation detection
[0065] Cell prolifera...
Embodiment 2
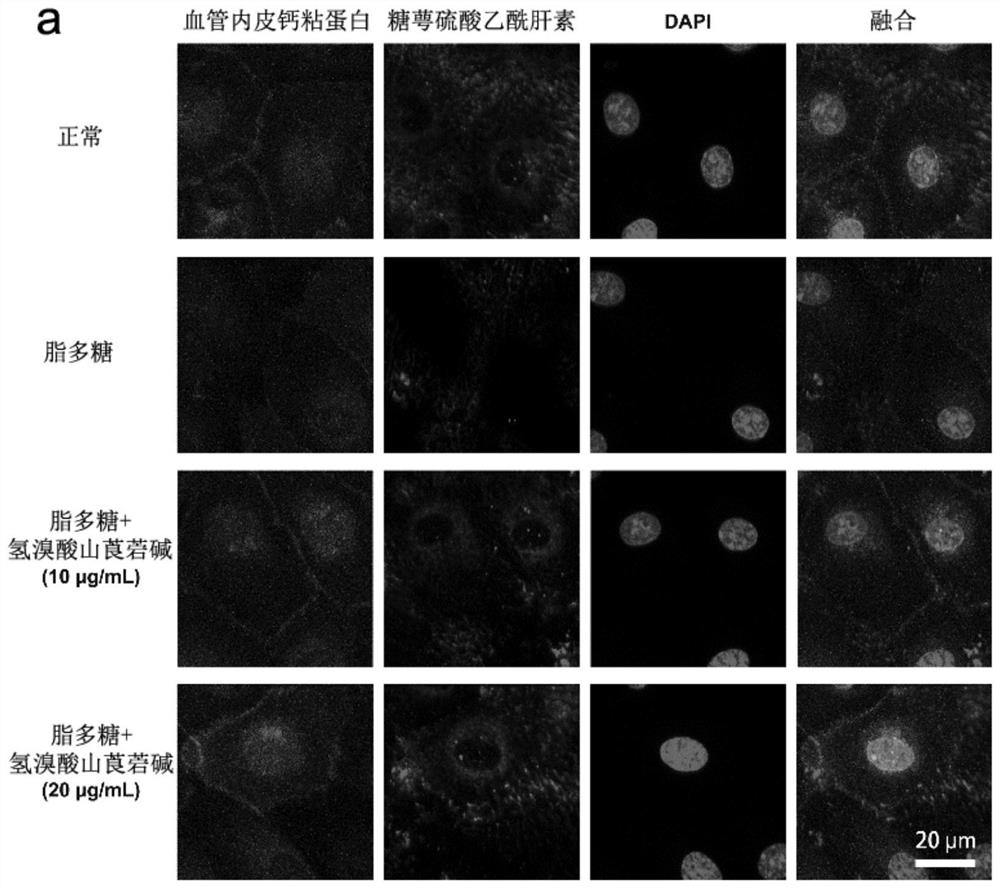
[0069] Example 2: Effect of anisodamine hydrobromide on glycocalyx and adhesion of vascular endothelial cells
[0070] 2.1 Experimental grouping: normal control group, LPS treatment group (50 μg / mL), low concentration administration group (LPS 50 μg / mL + anisodamine hydrobromide 10 μg / mL), high concentration administration group (LPS 50 μg / mL + hydrogen bromide Anisodamine 20 μg / mL).
[0071] 2.2 Immunofluorescence staining and confocal microscopy
[0072] After the cells were fixed and permeabilized, they were blocked with 2% goat serum, using mouse heparan sulfate (HS) monoclonal antibody (1:100, 10E4 epitope, AMS Biotechnology, UK) and rabbit cadherin polyclonal antibody (1:100 , A0734, Abclonal, China) and Alexa Fluor 488 goat anti-mouse (1:400, A11001, Thermo Fisher), Alexa Fluor 568 donkey anti-rabbit (1:400, A10042, Thermo Fisher) secondary antibodies to label the cells. Cell nuclei were labeled with DAPI and detected under a confocal microscope on LSM710 after mounti...
Embodiment 3
[0075] Example 3: Effect of anisodamine hydrobromide on LPS disrupted endothelial permeability
[0076] 3.1 Experimental grouping: same as Example 2
[0077] 3.2 Endothelial permeability assay
[0078] cells (5×10 4 Inoculate the upper well of Transwell (0.4 μm, Corning, USA) in 200 μL ECM, add 200 μL ECM to the lower well, and inoculate at 37°C and 5% CO 2 Incubate for 3 days in an incubator until 90% confluent. The medium in the upper wells was replaced with fresh ECM at 50 μg / mL LPS with or without anisodamine hydrobromide (10 or 20 μg / mL), and the medium in the lower wells was replaced with fresh ECM. After 24 hours, 10 μL of fluorescein isothiocyanate (FITC)-dextran (40 kDa; Cat. 46944, Sigma-Aldrich, USA) was added to the upper well. After 40 min, remove 100 µL of medium from the lower chamber. Measured fluorescence values at wavelengths of 490nm and 525nm. The leakage of FITC-dextran was calculated to assess the permeability of the endothelial cell layer.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com