Application of exosome in preparing medicine for treating interstitial lung injury and/or pulmonary fibrosis
A pulmonary fibrosis and exosome technology, which is applied in the field of medicine to achieve the effect of repairing lung epithelial tissue, treating interstitial lung injury and/or pulmonary fibrosis, and having broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
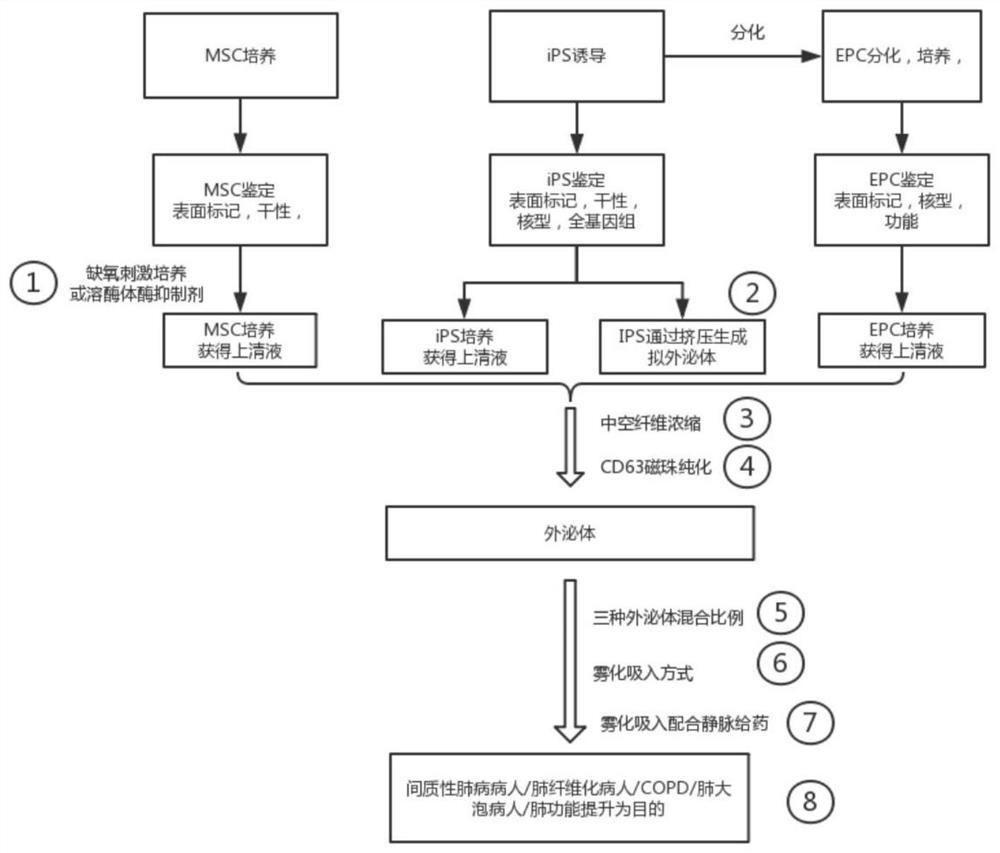
Image
Examples
Embodiment 1
[0058] Example 1: Preparation of iPSC cells
[0059] Collect donor peripheral blood mononuclear cells, electroporate the target plasmid combination to reprogram them, and obtain a large number of autologous iPSC cells after monoclonal expansion. The specific cultivation method is:
[0060] Step 1: Collect peripheral blood mononuclear cells (PBMC), after culture and expansion, conduct plasmid electroporation, transfer the reprogramming combination plasmids into fibroblasts or peripheral blood mononuclear cells, and then inoculate into culture plates at 37°C, 5% CO 2 Cultured in an incubator.
[0061] Step 2: Follow the culture procedure of peripheral blood mononuclear cell reprogramming culture, replace the complete reprogramming medium after 7 days, and start picking single clones (that is, clearly visible under the microscope) after iPSCs form larger clones, and transfer them to 24-well plates The iPSC cells were obtained through generation-by-generation expansion.
[006...
Embodiment 2
[0070] Embodiment 2: Preparation and identification of EPC
[0071] A combination of multiple induction factors is used to induce the differentiation of iPSC-GFP to form iEPC-GFP, and the primary or secondary iEPCs are used for functional experiments such as cell transplantation directly, or the primary iEPCs are frozen for future use to reduce the risk of mutation during culture.
[0072] Step 1: Take the iPSCs that have been prepared and identified correctly, inoculate them at a density of 2,000-20,000 cells / mL on a cell culture plate pretreated with Matrigel, and place the cell plate in 37°C, 5% CO 2 Cell culture incubator, stand overnight.
[0073] Step 2: One day after iPSC seeding, replace the mesoderm induction medium, and then incubate at 37°C, 5% CO 2 Incubate in a cell culture incubator and maintain for 3 days without changing the medium until the formation of mesoderm cells on the side plate.
[0074] Step 3: After the formation of mesoderm cells on the side plate...
Embodiment 3
[0082] Example 3: Culture and identification of umbilical cord-derived MSC cells
[0083] Obtain a fresh and healthy newborn umbilical cord from the operating room, rinse the umbilical cord with PBS, remove the blood vessels with scissors and tweezers, peel off the Wahrenheit tissue inside, and cut the obtained tissue to 1mm 3 size, add α-MEM medium, at 37°C, 5% CO 2 Cultivate in an incubator, the culture medium contains 10% FBS, 100U / mL penicillin, 100U / mL streptomycin, after the cells are congested, they are digested and passaged with 0.25% trypsin.
[0084] After digesting the primary cells, add PBS to adjust the cell concentration to 1×10 6 / mL, take 200 μL of cell suspension and add 5 μL of fluorescently labeled monoclonal antibodies (CD29-FITC, CD34-FITC, CD44-FITC, CD45-CY5, CD105-PE, and CD106-PE) according to the combination plan, mix well, and keep away from room temperature. Incubate with light for 20 min and detect in flow cytometer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com