Preparation method of adipic acid dialkyl ester
A technology of dialkyl adipate and adipic acid, which is applied in the field of preparation of dialkyl adipate, can solve the problems of strong corrosion of sulfuric acid, long reaction time, and many side reactions, and achieve good selectivity , The preparation method is simple, the effect of the preparation is convenient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
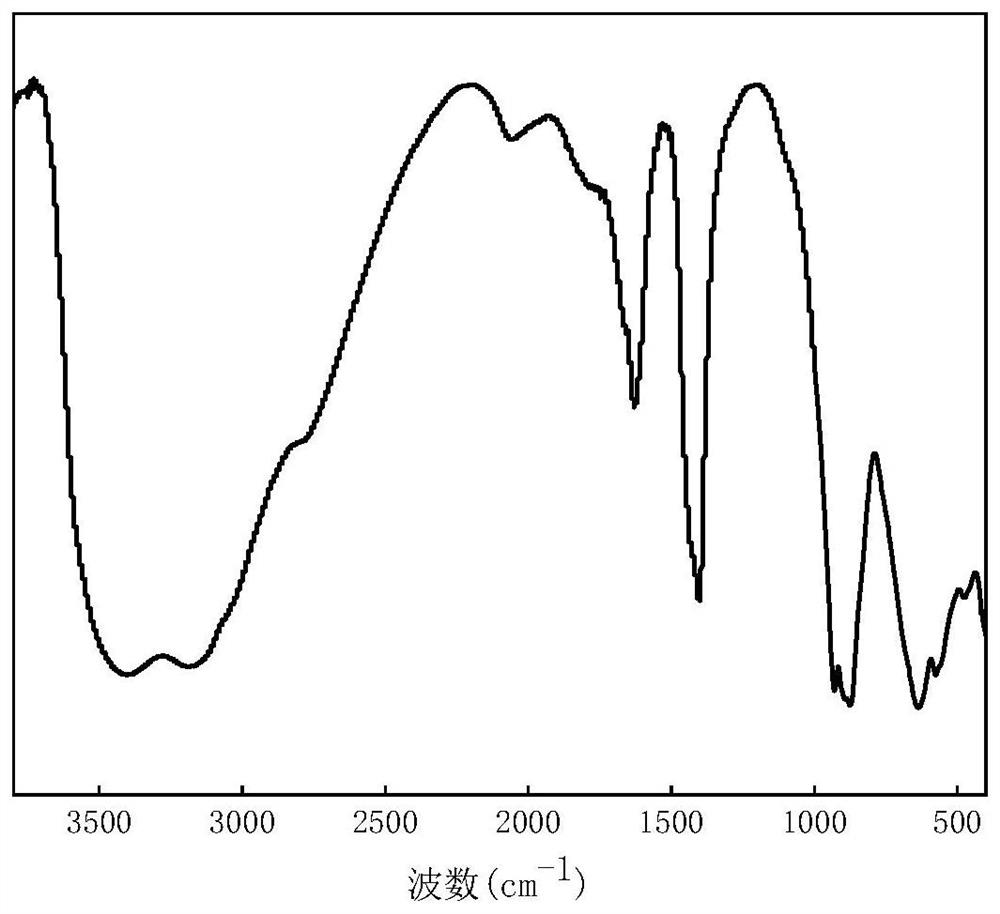
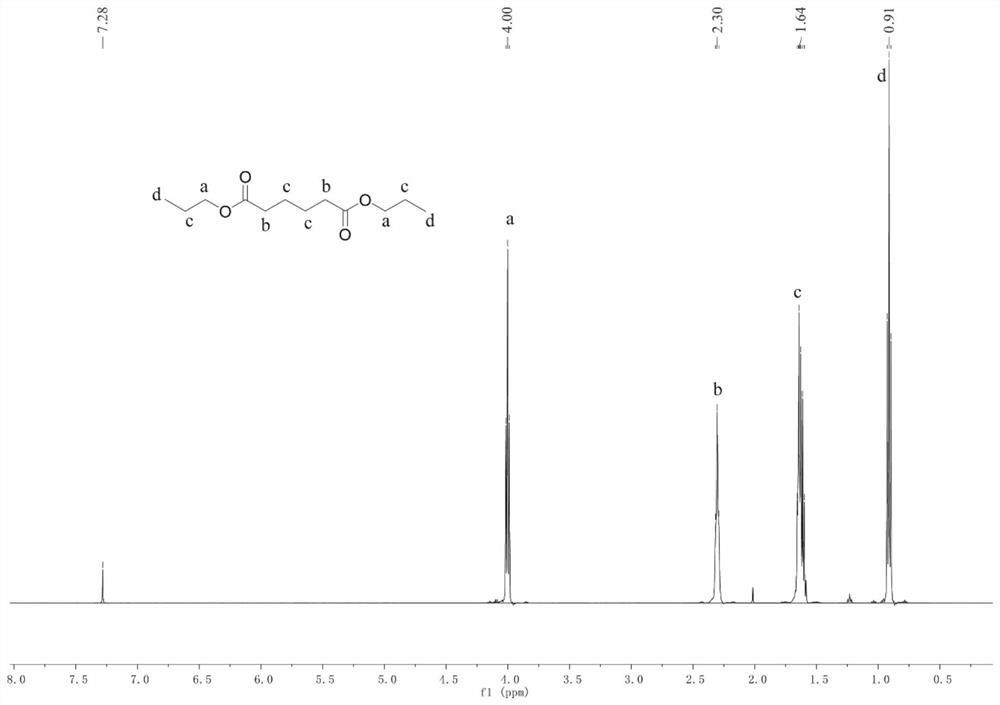
[0029] 1.46 g (0.01 mol) of adipic acid, 2.4 g (0.04 mol) of propanol, and 0.030 g (0.25 mol %) of [Cu Ⅱ Mo 6 ] Anderson-type heteropolyacids (such as figure 1 ), put it into a dry reaction test tube, the reaction temperature was controlled at 100 ° C, and the reaction was incubated for 8 hours. After the reaction was completed, it was cooled to room temperature, filtered, and the solution was washed with an aqueous sodium carbonate solution, and the organic phase was separated. The result is as figure 2 shown. The yield of the product dipropyl adipate was 62%.
Embodiment 2
[0031] 1.46g (0.01mol) of adipic acid, 2.96g (0.04mol) of butanol, and 0.060g (0.5mol%) of [Cu Ⅱ Mo 6 ] Anderson-type heteropolyacids (such as figure 1 ), put it into a dry reaction test tube, the reaction temperature was controlled at 120 ° C, and the reaction was incubated for 12 h. After the reaction was completed, it was cooled to room temperature, filtered, and the solution was washed with sodium carbonate aqueous solution, and the organic phase was separated. After concentration and drying, nuclear magnetic testing was carried out. The yield of the product dibutyl adipate was 67%.
Embodiment 3
[0033] 2.92 g (0.02 mol) of adipic acid, 7.80 g (0.06 mol) of 1-octanol, and 0.12 g (0.5 mol %) of [Cu Ⅱ Mo 6 ] Anderson-type heteropolyacids (such as figure 1 ), put it into a dry reaction test tube, the reaction temperature was controlled at 140 ° C, and the reaction was incubated for 4 h. After the reaction was completed, it was cooled to room temperature, warmed, filtered, the solution was washed with sodium carbonate aqueous solution, and the organic phase was separated. After concentration and drying, NMR test. The yield of the product dioctyl adipate was 58%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com