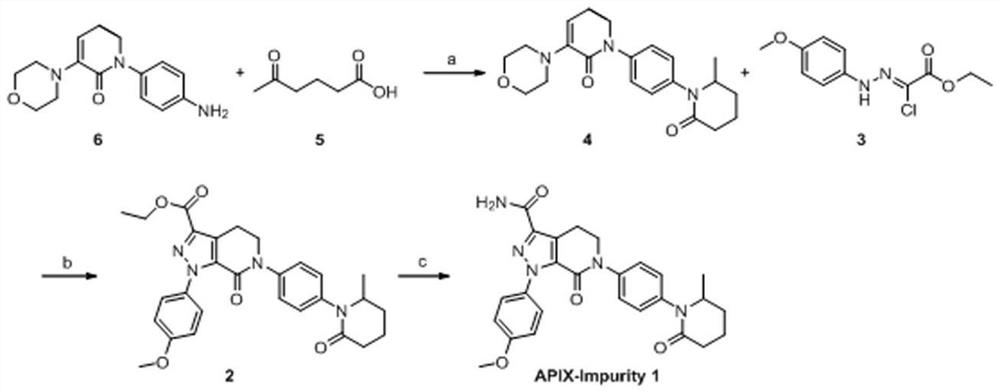
Preparation method of apixaban impurity 1
A technology for apixaban and impurities is applied in the field of drug impurity synthesis to achieve the effects of high reaction yield, good yield and high impurity purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Compound 5,6-dihydro-3-(4-morpholinyl)-1-[4-(2-oxo-6-methyl-1-piperidinyl)phenyl]-2(1H)-pyridinone The preparation of: Add compound 1-(4-aminophenyl)-5,6-dihydro-3-(4-morpholinyl)-2(1H)-pyridone (0.1mol, 27.84g ), compound 5-oxohexanoic acid (0.1mol), aluminum trichloride (0.05mol) and phenylsilane (0.3mol), heated to 40°C for 12h, after the reaction was completed, cooled to room temperature, and the reaction solution Quench to 150mL of water, extract twice with 100mL*2 dichloromethane, combine the organic phase, concentrate the organic phase to leave a volume of about 50mL, add 160mL of n-hexane, cool down to about 10°C and stir for 3h, filter to obtain yellow to off-white The solid is the compound 5,6-dihydro-3-(4-morpholinyl)-1-[4-(2-oxo-6-methyl-1-piperidinyl)phenyl]-2(1H) - Pyridone (product: 35.32 g, yield: 91.8%).
Embodiment 2
[0026] Compound 1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxo-6-methylpiperidin-1-yl)phenyl]-4,5,6, Preparation of ethyl 7-tetrahydro-1H-pyrazol[3,4-c]pyridine-3-carboxylate: add the compound [(4-methoxyphenyl)hydrazino] ethyl chloroacetate sequentially to the reaction flask Ester (0.08mol, 20.54g) and compound 5,6-dihydro-3-(4-morpholinyl)-1-[4-(2-oxo-6-methyl-1-piperidinyl)phenyl ]-2(1H)-pyridone (0.08mol, 30.68g), heat up to 90-95°C, add triethylamine (0.16mol, 16.2g) dropwise, after the dropwise addition, continue to react for 3 hours, cool down to room temperature , add dilute hydrochloric acid dropwise, after the dropwise addition, cool down to about 10°C and stir for 2 hours, filter, wash the filter cake with water several times to obtain a yellow solid which is the compound 1-(4-methoxyphenyl)-7-oxo Substituent-6-[4-(2-oxo-6-methylpiperidin-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazol[3,4-c] Ethyl pyridine-3-carboxylate (product: 29.74 g, yield: 74.0%).
Embodiment 3
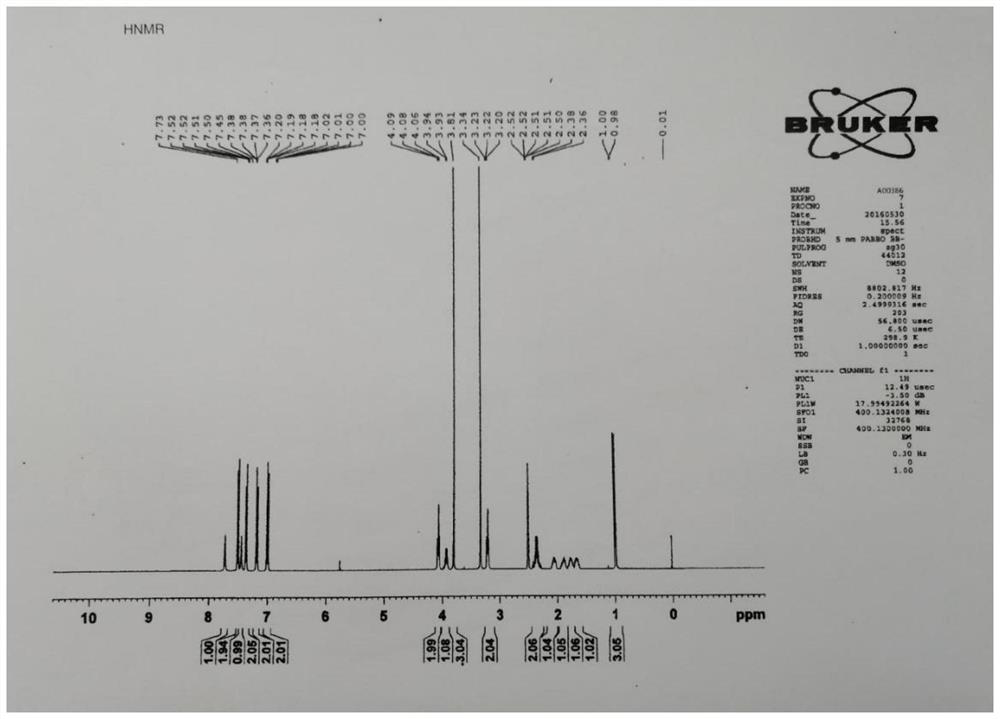
[0028] Preparation of Apixaban impurity 1: add compound 1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxo-6-methylpiperidine-1) to the reaction flask -yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazol[3,4-c]pyridine-3-carboxylic acid ethyl ester (0.02mol, 10.05g) was dissolved in 50mL tetrahydrofuran, added 30% ammonia solution (0.4mol, 23mL), seal the reaction vessel, raise the temperature to 80°C and react for 6h. Stir at about 10°C for 2 hours, filter to obtain an off-white solid that is apixaban impurity 1 (product: 8.33g, yield: 88.0%; purity: 99.2%), ESI: m / z[M+H] + 474.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com