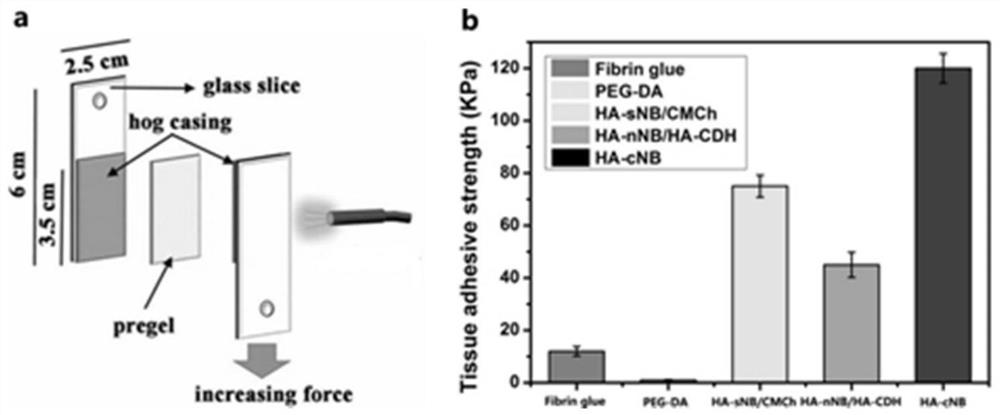
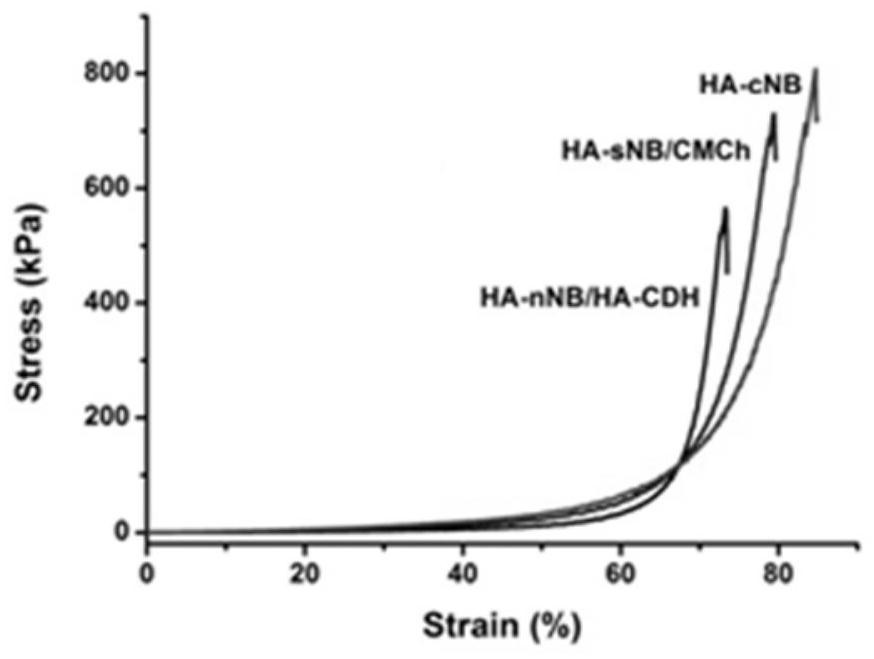
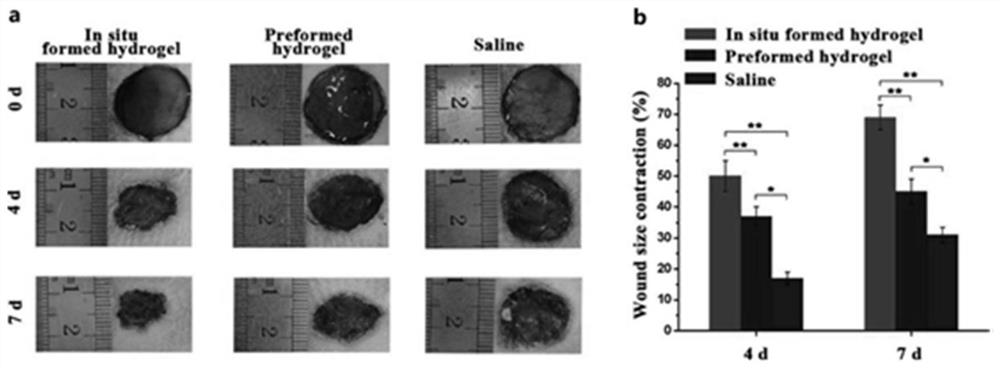
Preparation, raw material, product and application of optical coupling synergistic cross-linked hydrogel material
A technology of optical trigger and halogen atom, applied in the field of preparation of photocoupling synergistic cross-linking hydrogel materials, can solve the problems of limiting the clinical transformation of non-radical photo-coupling cross-linking technology, unfavorable clinical operation, slowing down of cross-linking speed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0247] Embodiment one: the synthesis of component A-1
[0248]
[0249] (1) Synthesis of compound 1: synthesized according to the method disclosed in references Kunihiko Morihiro.; Tetsuya Kodama.; Shohei Mori.; Satoshi Obika.Org.Biomol.Chem.2014,12,2468. 1 H NMR (400MHz, CDCl 3 ):δ=7.71(s,1H),7.22(s,1H),4.03(s,2H),4.13(t,J=6.1Hz,2H),3.99(s,3H),3.32(dd,J= 11.6,5.7Hz,2H),2.82(t,J=5.9Hz,2H),2.44(t,J=7.2Hz,2H),2.26-2.17(m,2H). MS(ESI):[M+H ] 344.1207.
[0250] (2) Synthesis of component A-1: dissolve hyaluronic acid (2g, 340kDa) in 100mL of 0.01mol / L 2-(N-morpholine)ethanesulfonic acid MES buffer solution (pH=5.2), stir Until it is completely dissolved, weigh compound 1 (69mg, 0.2mmol) and dissolve it in 10mL dimethyl sulfoxide DMSO, add the above reaction solution, weigh 4-(4,6-dimethoxytriazin-2-yl)- 4-Methylmorpholine hydrochloride DMTMM (0.4g, 1.5mmol) was dissolved in 3mL MES buffer solution, added to the above reaction solution three times (every 1h), and reacted a...
Embodiment 2
[0251] Embodiment two: the synthesis of component A-2
[0252]
[0253] (1) Synthesis of compound 2: according to the method disclosed in the references Yunlong Yang; Jieyuan Zhang; Zhenzhen Liu; Qiuning Lin; Xiaolin Liu; Chunyan Bao; Yang Wang; Linyong Zhu.Adv.Mater.2016,28,2724. .
[0254] (2) Synthesis of compound 3: Dissolve compound 2 (1g, 3.0mmol) in 50mL tetrahydrofuran, add carbon tetrabromide CBr 4 (2g, 6.0mmol), and triphenylphosphine PPh 3 (1.6g, 6.0mmol), under the protection of argon, stirred at room temperature for 2h, after the reaction, 5mL of water was added to quench the reaction, the solvent was spin-dried, extracted with ethyl acetate, and separated by column chromatography (PE:DCM=4:1) , to obtain compound 3 (1.0 g, yield 84%). 1 H NMR (400MHz, CDCl 3 ): δ=7.71(s,1H),7.22(s,1H),4.56(s,2H),4.13(t,J=6.1Hz,2H),3.99(s,3H),3.32(dd,J= 11.6,5.7Hz,2H),2.82(t,J=5.9Hz,2H),2.44(t,J=7.2Hz,2H),2.26-2.17(m,2H).MS (ESI):[M+H ] 390.0623.
[0255] (3) Synthesis o...
Embodiment 3
[0257] Embodiment three: the synthesis of component A-3
[0258]
[0259] (1) Synthesis of compound 5: synthesized according to the method disclosed in references James F.Cameron.; JeanM.J.Frechet.J.Am.Chem.Soc.1991,113,4303.
[0260] (2) Synthesis of Compound 6: According to the method of Example 2, Compound 6 was prepared from Compound 5 (yield 73%). 1 H NMR (400MHz, CDCl 3 ):δ=7.71(s,1H),7.22(s,1H),4.66(m,1H),4.13(t,J=6.1Hz,2H),3.99(s,3H),3.32(dd,J= 11.6,5.7Hz,2H),2.82(t,J=5.9Hz,2H),2.44(t,J=7.2Hz,2H),2.26-2.17(m,2H),1.33(d,J=6.9Hz, 3H).MS(ESI):[M+H]404.0863.
[0261] (3) Synthesis of Compound 7: According to the method of Example 2, Compound 7 was prepared from Compound 6 (yield 70%). 1 H NMR (400MHz, CDCl 3 ):δ=7.71(s,1H),7.22(s,1H),4.86(m,1H),4.42(m,1H),4.13(t,J=6.1Hz,2H),3.99(s,3H) ,3.95(s,3H),3.43(d,J=5.6,2H),3.32(dd,J=11.6,5.7 Hz,2H),2.82(t,J=5.9Hz,2H),2.44(t,J =7.2Hz,2H),2.26-2.17(m,2H),1.42(s,9H), 1.33(d,J=6.9Hz,3H).MS(ESI):[M+H]559.2402.
[0262] (4) Syn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com