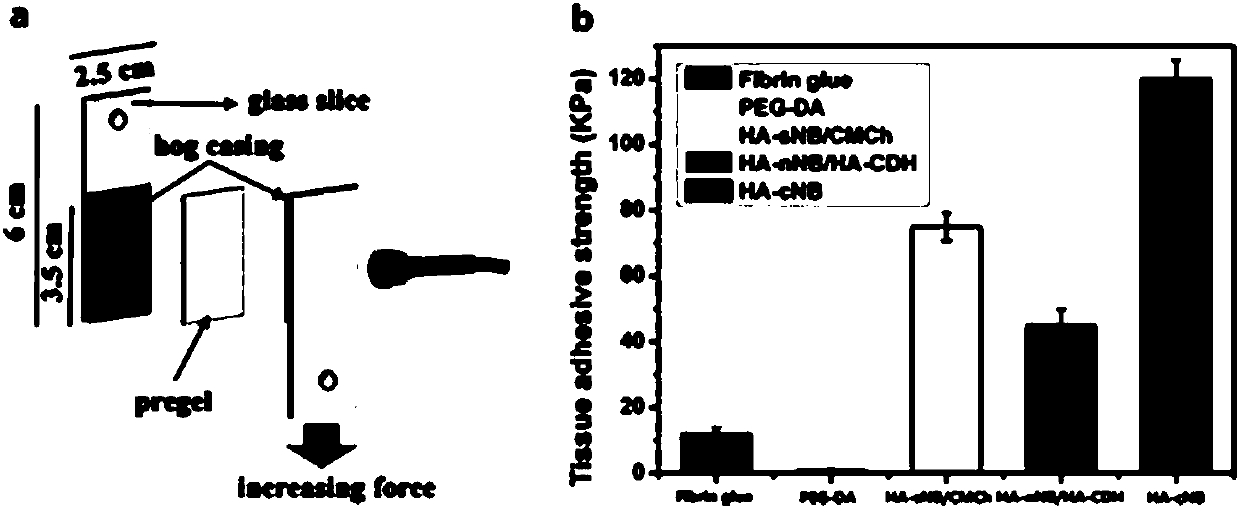
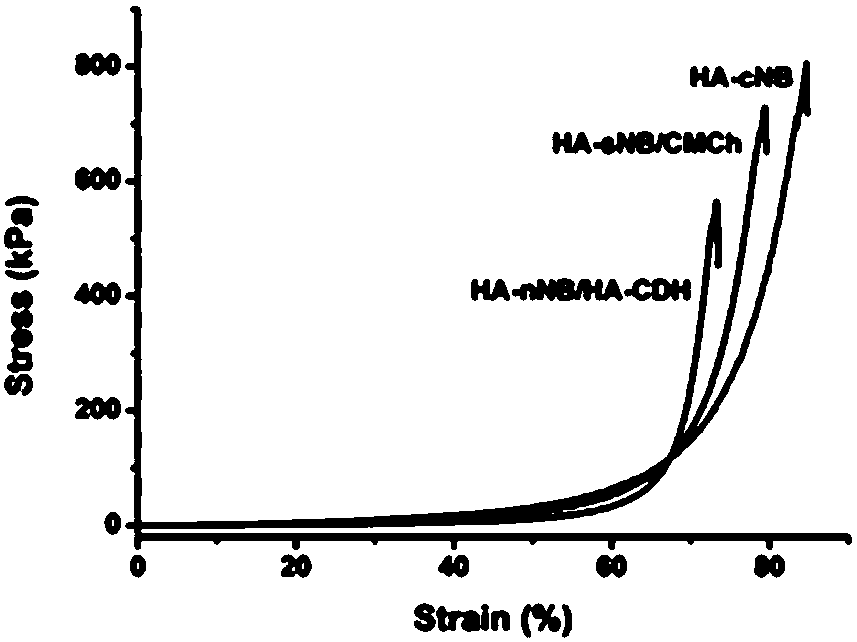
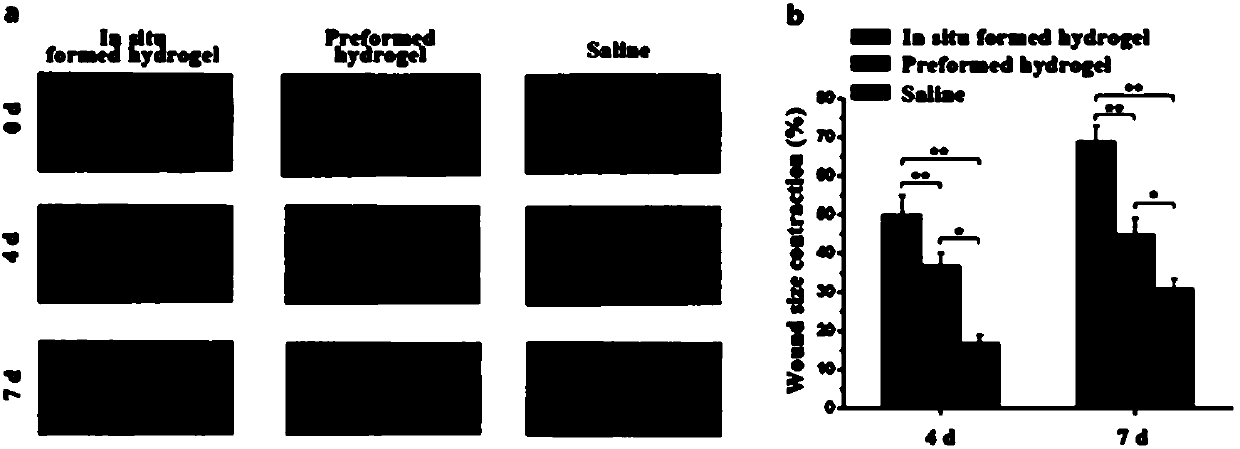
Preparation, raw material, product and application of photo-coupling co-crosslinking hydrogel material
A technology of photo-trigger and halogen atoms, which is applied in the field of preparation of photocoupling synergistically crosslinked hydrogel materials, can solve the problems of unfavorable clinical operation, limiting the clinical transformation of non-free radical photocoupling crosslinking technology, slowing down of crosslinking speed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0245] Embodiment one: the synthesis of component A-1
[0246]
[0247] (1) Synthesis of compound 1: synthesized according to the method disclosed in references Kunihiko Morihiro.; Tetsuya Kodama.; Shohei Mori.; Satoshi Obika.Org.Biomol.Chem.2014,12,2468. 1 H NMR (400MHz, CDCl 3 ):δ=7.71(s,1H),7.22(s,1H),4.03(s,2H),4.13(t,J=6.1Hz,2H),3.99(s,3H),3.32(dd,J= 11.6,5.7Hz,2H),2.82(t,J=5.9Hz,2H),2.44(t,J=7.2Hz,2H),2.26-2.17(m,2H). MS(ESI):[M+H ] 344.1207.
[0248] (2) Synthesis of component A-1: dissolve hyaluronic acid (2g, 340kDa) in 100mL of 0.01mol / L 2-(N-morpholine)ethanesulfonic acid MES buffer solution (pH=5.2), stir Until it is completely dissolved, weigh compound 1 (69mg, 0.2mmol) and dissolve it in 10mL dimethyl sulfoxide DMSO, add the above reaction solution, weigh 4-(4,6-dimethoxytriazin-2-yl)- 4-Methylmorpholine hydrochloride DMTMM (0.4g, 1.5mmol) was dissolved in 3mL MES buffer solution, added to the above reaction solution three times (every 1h), and reacted a...
Embodiment 2
[0249] Embodiment two: the synthesis of component A-2
[0250]
[0251] (1) Synthesis of compound 2: according to the method disclosed in the references Yunlong Yang; Jieyuan Zhang; Zhenzhen Liu; Qiuning Lin; Xiaolin Liu; Chunyan Bao; Yang Wang; Linyong Zhu.Adv.Mater.2016,28,2724. .
[0252] (2) Synthesis of compound 3: Dissolve compound 2 (1g, 3.0mmol) in 50mL tetrahydrofuran, add carbon tetrabromide CBr 4 (2g, 6.0mmol), and triphenylphosphine PPh 3 (1.6g, 6.0mmol), under the protection of argon, stirred at room temperature for 2h, after the reaction, 5mL of water was added to quench the reaction, the solvent was spin-dried, extracted with ethyl acetate, and separated by column chromatography (PE:DCM=4:1) , to obtain compound 3 (1.0 g, yield 84%). 1 H NMR (400MHz, CDCl 3 ): δ=7.71(s,1H),7.22(s,1H),4.56(s,2H),4.13(t,J=6.1Hz,2H),3.99(s,3H),3.32(dd,J= 11.6,5.7Hz,2H),2.82(t,J=5.9Hz,2H),2.44(t,J=7.2Hz,2H),2.26-2.17(m,2H).MS (ESI):[M+H ] 390.0623.
[0253] (3) Synthesis o...
Embodiment 3
[0255] Embodiment three: the synthesis of component A-3
[0256]
[0257] (1) Synthesis of compound 5: synthesized according to the method disclosed in references James F.Cameron.; JeanM.J.Frechet.J.Am.Chem.Soc.1991,113,4303.
[0258] (2) Synthesis of Compound 6: According to the method of Example 2, Compound 6 was prepared from Compound 5 (yield 73%). 1 H NMR (400MHz, CDCl 3 ):δ=7.71(s,1H),7.22(s,1H),4.66(m,1H),4.13(t,J=6.1Hz,2H),3.99(s,3H),3.32(dd,J= 11.6,5.7Hz,2H),2.82(t,J=5.9Hz,2H),2.44(t,J=7.2Hz,2H),2.26-2.17(m,2H),1.33(d,J=6.9Hz, 3H).MS(ESI):[M+H]404.0863.
[0259] (3) Synthesis of Compound 7: According to the method of Example 2, Compound 7 was prepared from Compound 6 (yield 70%). 1 H NMR (400MHz, CDCl 3 ):δ=7.71(s,1H),7.22(s,1H),4.86(m,1H),4.42(m,1H),4.13(t,J=6.1Hz,2H),3.99(s,3H) ,3.95(s,3H),3.43(d,J=5.6,2H),3.32(dd,J=11.6,5.7 Hz,2H),2.82(t,J=5.9Hz,2H),2.44(t,J =7.2Hz,2H),2.26-2.17(m,2H),1.42(s,9H), 1.33(d,J=6.9Hz,3H).MS(ESI):[M+H]559.2402.
[0260] (4) Syn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com