Fluorescent probe capable of simultaneously recognizing H2S and HSO3 <-> through near-infrared emission and application thereof
An HSO3-, fluorescent probe technology, applied in luminescent materials, fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of few fluorescent probes, complex synthesis, short emission wavelength, etc., to achieve easy separation and purification, and simple synthesis process , the effect of good sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The specific synthesis steps of fluorescent probe L are as follows:
[0038]
[0039] Compound 1 (374.4 mg, 1.0 mmol), triethylamine (101.0 mg, 1.0 mmol) was dissolved in dry CH 2 Cl 2 2,4-Dinitrobenzenesulfonyl chloride (266.6 mg, 1.0 mmol) was slowly added dropwise under stirring in an ice bath, and then stirred at room temperature for 8 hours. After the reaction was completed, the obtained crude product was purified by thin-layer column chromatography, using EA:PE=1:10~1:3 (v / v) as gradient elution to obtain 302.07 mg of probe L, with a yield of 50%.
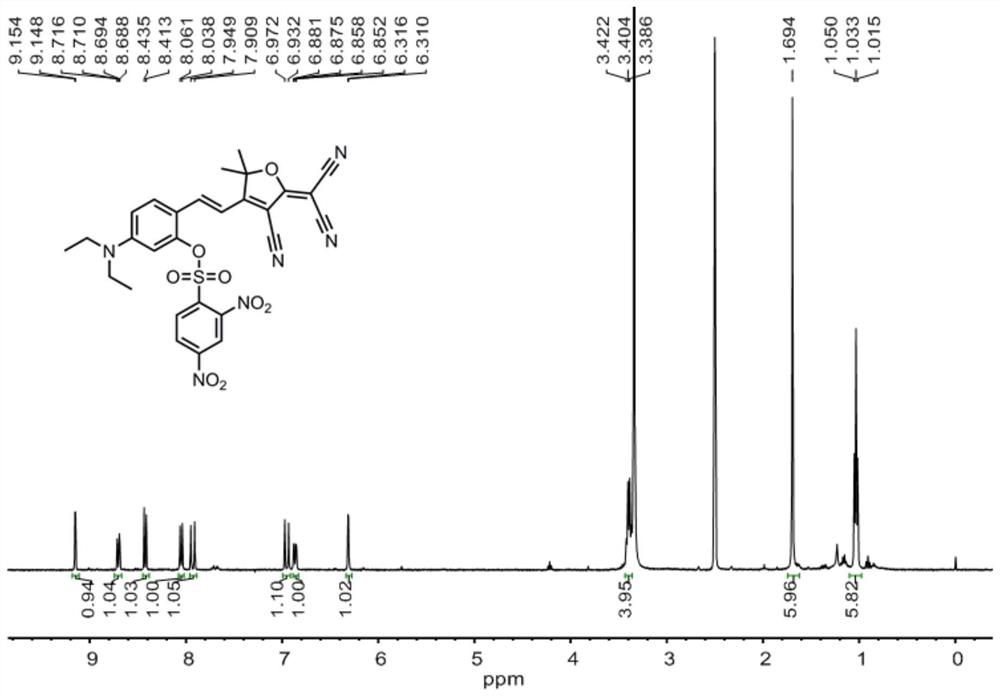
[0040] 1 H NMR (400MHz, DMSO-d 6 )δ9.15(d, J=2.2Hz, 1H), 8.70(dd, J=8.6, 2.0Hz, 1H), 8.42(d, J=8.7Hz, 1H), 8.05(d, J=9.3Hz, 1H), 7.93(d, J=15.9Hz, 1H), 6.95(d, J=15.9Hz, 1H), 6.87(d, J=7.4Hz, 1H), 6.31(d, J=2.1Hz, 1H) ,3.39(dd,J=14.0,7.0Hz,4H),1.69(s,6H),1.03(t,J=7.0Hz,6H).
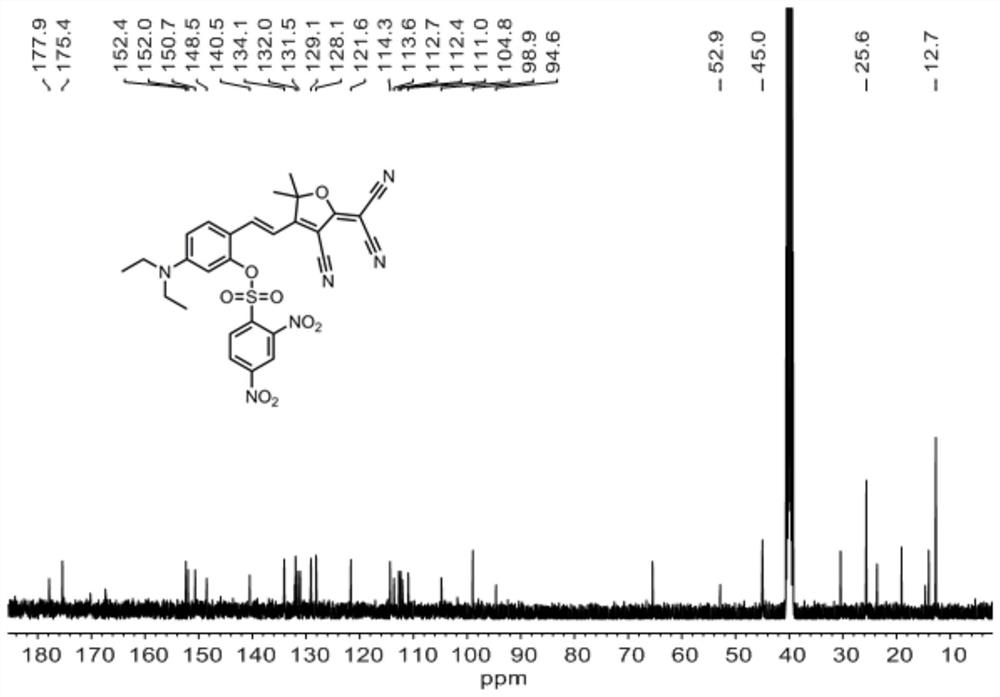
[0041] 13 C NMR (101MHz, DMSO-d 6 )δ177.87, 140.51, 134.07, 131.12, 129.09, 128.14, 112.38, 44.99, 40.56, 40.35, 40.14, 39.93, 39.72, ...
Embodiment 2
[0044] The specific synthesis steps of fluorescent probe L are as follows:
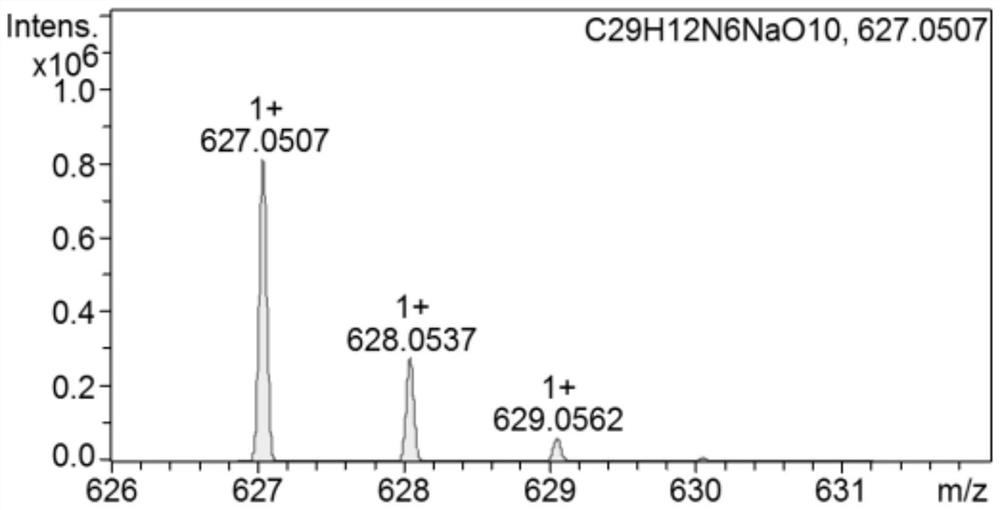
[0045] Compound 1 (374.4 mg, 1.0 mmol), triethylamine (202.0 mg, 2 mmol) was dissolved in dry CH 2 Cl 2 2,4-Dinitrobenzenesulfonyl chloride (399.9 mg, 1.5 mmol) was slowly added dropwise under stirring in an ice bath, followed by stirring at room temperature for 16 hours. After the end, the crude product was spin-dried to obtain the crude product. The crude product was purified by thin-layer column chromatography, and 392.67 mg of probe L was obtained with a yield of 65% using EA:PE=1:10~1:3 (v / v) for gradient elution. . The fluorescent probe L of this embodiment 1 H NMR spectrum as figure 1 , 13 C NMR spectrum as figure 2 , high-resolution mass spectrometry such as image 3 shown.
Embodiment 3
[0047] The specific synthesis steps of fluorescent probe L are as follows:
[0048] Compound 1 (374.4 mg, 1.0 mmol), triethylamine (303.0 mg, 3 mmol), dissolved in dry CH 2 Cl 2 2,4-Dinitrobenzenesulfonyl chloride (533.2mg, 2mmol) was slowly added dropwise under stirring in an ice bath, and then stirred at room temperature for 24 hours. After the reaction was completed, the solvent was spin-dried, and the obtained crude product was purified by thin-layer column chromatography, and EA:PE=1:0~1:3 (v / v) was used as gradient elution to obtain 362.48mg of probe L, with a yield of 60% . The fluorescent probe L of this embodiment 1 H NMR spectrum as figure 1 , 13 C NMR spectrum as figure 2 , high-resolution mass spectrometry such as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com