A kind of blue fluorescent silver nanocluster and its preparation method and application
A silver nanocluster, blue fluorescence technology, applied in nanotechnology, nano-optics, nanotechnology and other directions, can solve the problems of low sensitivity, low selectivity, complex operation, etc., achieve high quantum yield, short reaction time, The effect of accurate detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
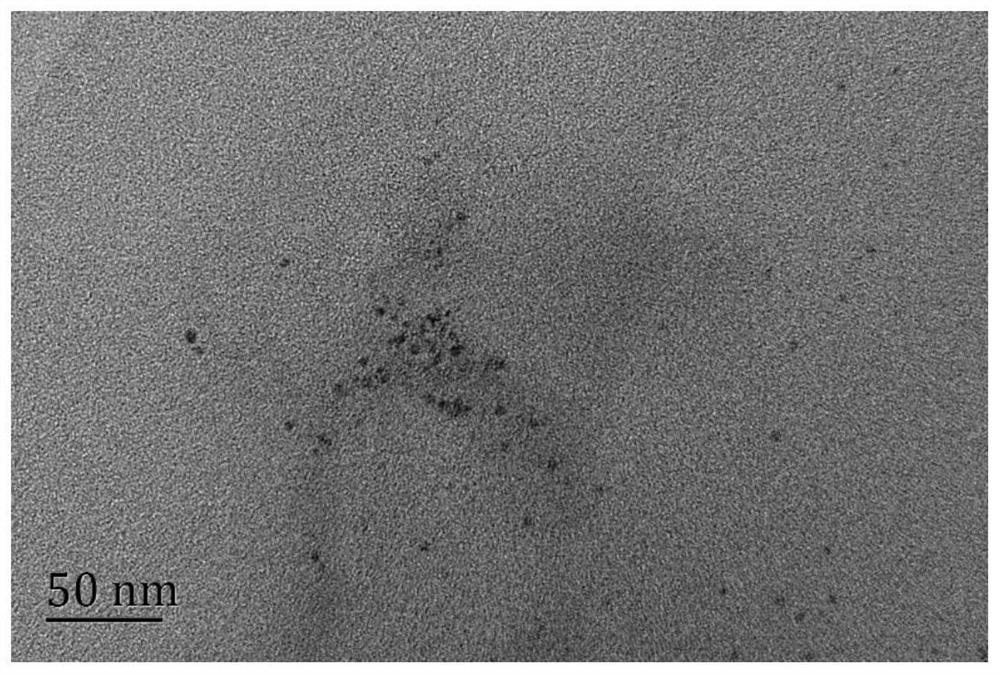
[0040] Mix 100mL 0.1mol / L histidine aqueous solution and 1mL 0.25mol / L silver nitrate solution at room temperature (n 硝酸银 :n 组氨酸 =1:40), after magnetic stirring for 10 minutes, react in a microwave oven with a power of 700W for 4 minutes, and the reaction mixture was dialyzed for 24 hours using a dialysis membrane with a molecular mass of 500da to obtain fluorescent silver nanoclusters.
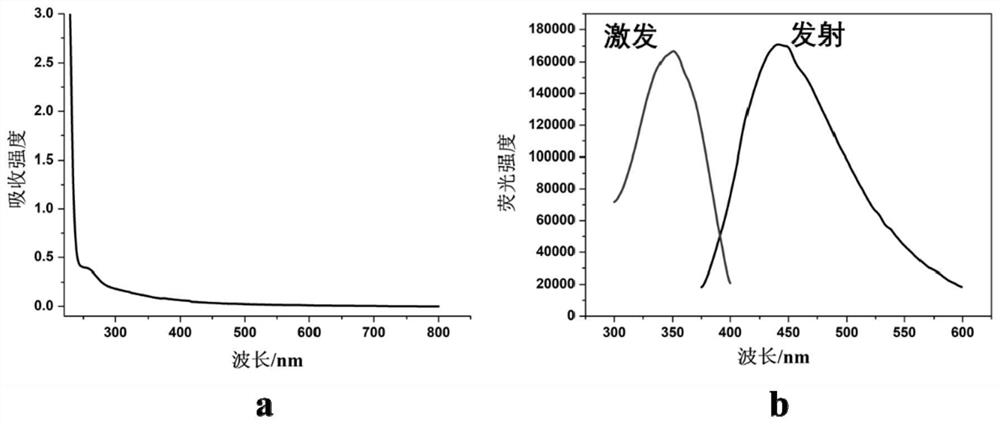
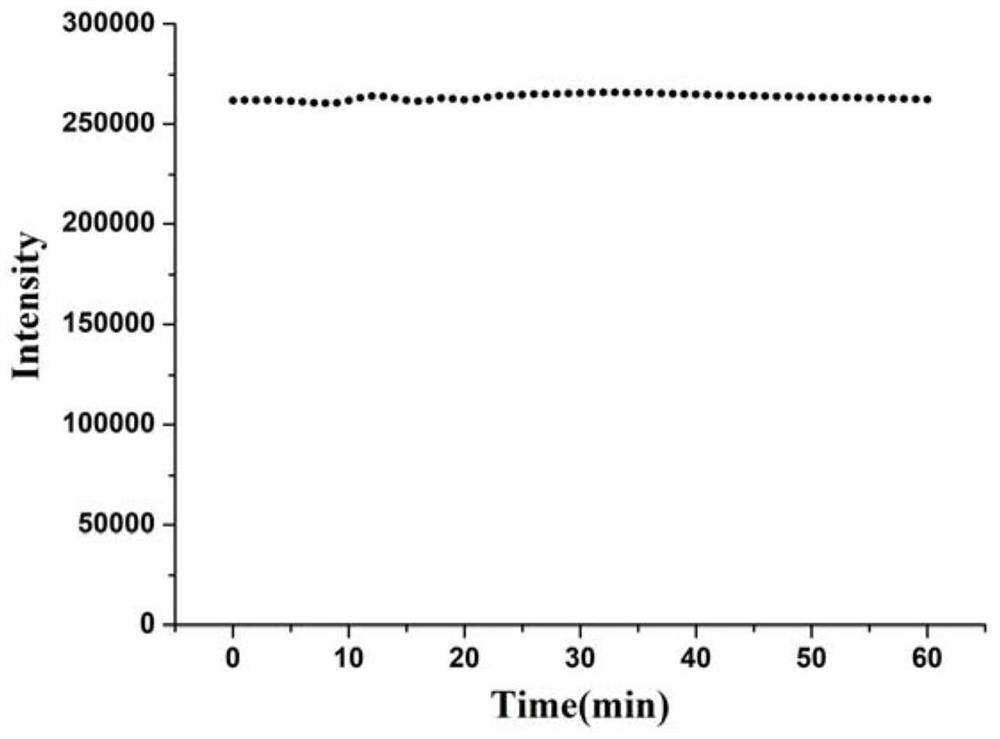
[0041] The fluorescence emission peak of the silver nanocluster is around 450nm, and when observed with a black background under an ultraviolet lamp, it presents blue fluorescence with a fluorescence intensity of 4.27×10 4 , with a quantum yield of 1.3%. The stability of the luminescence intensity at 450nm within 60 minutes was detected, and the fluorescence intensity value remained basically unchanged after 60 minutes, indicating that the silver nanocluster has good photobleaching resistance.
Embodiment 2
[0043] Mix 100mL 0.1mol / L histidine aqueous solution and 1mL 0.25mol / L silver nitrate solution at room temperature (n 硝酸银 :n 组氨酸 =1:40), after magnetic stirring for 10 minutes, react in a microwave oven with a power of 700W for 8 minutes, and the reaction mixture was dialyzed for 24 hours using a dialysis membrane with a molecular mass of 500da to obtain fluorescent silver nanoclusters.
[0044] The fluorescence emission peak of the silver nanocluster is around 450nm, and when observed with a black background under an ultraviolet lamp, it presents blue fluorescence with a fluorescence intensity of 6.15×10 4 , with a quantum yield of 1.8%. The stability of the luminescence intensity at 450nm within 60 minutes was detected, and the fluorescence intensity value remained basically unchanged after 60 minutes, indicating that the silver nanocluster has good photobleaching resistance.
Embodiment 3
[0046] Mix 100mL 0.125mol / L histidine aqueous solution and 1mL 0.25mol / L silver nitrate solution at room temperature (n 硝酸银 :n 组氨酸 =1:50), after magnetic stirring for 10min, react in a microwave oven with a power of 700W for 4min, and the reaction mixture was dialyzed for 24 hours using a dialysis membrane with a molecular mass of 500da to obtain fluorescent silver nanoclusters.
[0047] The fluorescence emission peak of the silver nanocluster is around 440nm, and when observed with a black background under an ultraviolet lamp, it presents blue fluorescence with a fluorescence intensity of 7.94×10 4 , with a quantum yield of 2.4%. The stability of the luminescence intensity at 440nm within 60 minutes was detected, and the fluorescence intensity value remained basically unchanged after 60 minutes, indicating that the silver nanocluster has good photobleaching resistance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com