Small molecule triple immunochromatography detection method, test strip and kit
An immunochromatographic detection and small molecule technology, applied in analytical materials, biological testing, measurement devices, etc., can solve the problems of many influencing conditions, limited detection sensitivity, difficult to control accurately, etc., to improve detection sensitivity and wide range of detection applications. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] This embodiment takes the detection of diazepam as an example to illustrate the preparation of the detection test strip of the present invention. The modified protein was selected from BSA, and the signal substance of the labeled antibody was selected from carboxylated fluorescent quantum dot microspheres (QB, purchased from Beijing Najing Biotechnology Co., Ltd., product model: QBB12117, H07187M).
[0056] The labeled antigen signal substance is selected from the Raman signal surface enhancement material Au@Ag-R, which is prepared by the following method: reducing chloroauric acid to gold seeds by sodium citrate, further reducing silver nitrate by sodium citrate reduction method and coating on Gold seeds to realize the preparation of Au@Ag. After the preparation of the material is completed, a Raman reporter molecule dissolved in 5% methanol aqueous solution is added at a concentration of 10 mg / mL; the Raman reporter molecule is DTNB: 5,5'-dithiobis(2-nitrobenzoic acid...
Embodiment 2
[0064] The present embodiment takes the detection of diazepam as an example to illustrate the detection method of the present invention, test with the test strip that embodiment 1 makes:
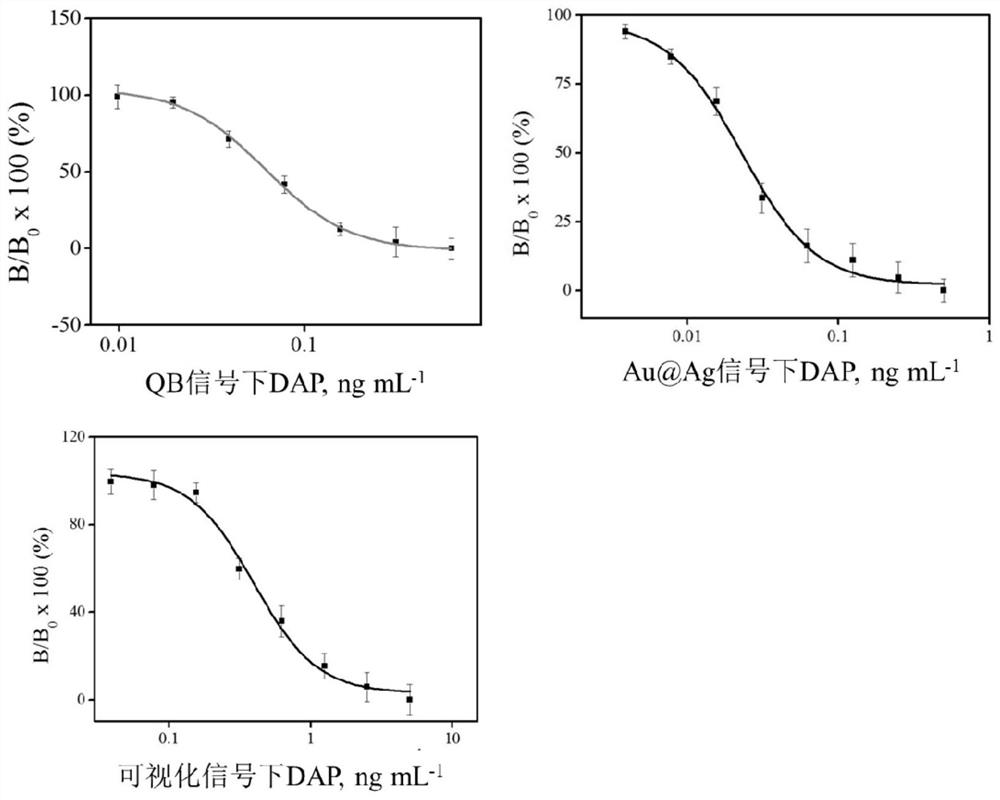
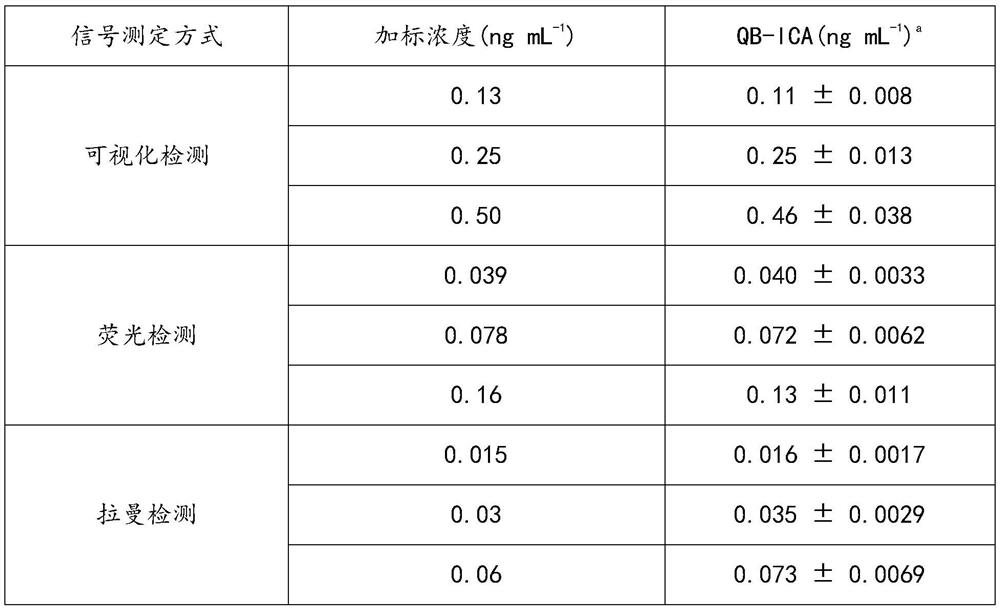
[0065] Prepare standard curve
[0066] Diazepam (DAP) standard was prepared as DAP solutions with different concentrations in phosphate buffer: 1.28ng / mL, 0.64ng / mL, 0.32ng / mL, 0.16ng / mL, 0.08ng / mL, 0.04ng / mL, 0.02ng / mL, 0.01ng / mL. The composition of the phosphate buffer is as follows: based on 0.05mol / L phosphate buffer, the following substances are added in the following weight percentages: 2% sucrose, 5% fructose, 1% PEG, 3% Tween-20.
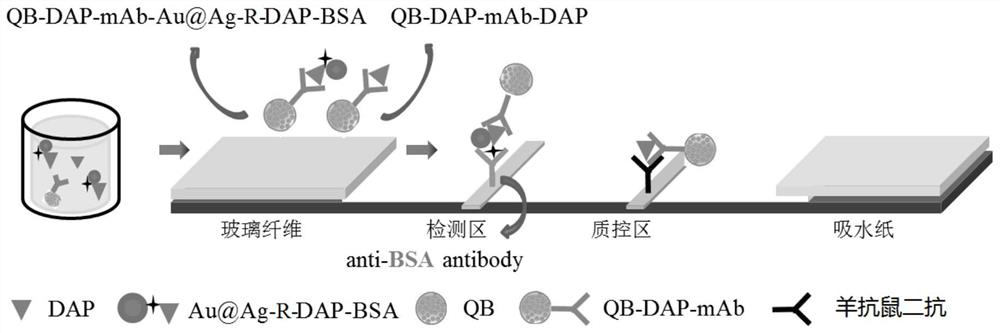
[0067] Au@Ag-R-DAP-BSA 0.1μg / 100μL solution, QB-DAP-mAb were mixed with different concentrations of DAP standards at a dilution ratio of 1:3000, QB-DAP-mAb and Au@Ag-R-DAP -The final concentration range of BSA is 0.2-0.8mg / mL and 0.01-0.04mg / mL respectively. At 37℃, DAP was incubated with labeled antibody QB-DAP-mAb and Au@Ag-R-DAP-BSA for 10min, A mix...
Embodiment 3
[0073] This embodiment takes DAP in aquatic products as an example to illustrate that the sample to be tested is detected by the method of the present invention, and the steps are as follows:
[0074] Sample to be tested: DAP was added to fish meat;
[0075] The test strip prepared in Example 1 was equilibrated to room temperature.
[0076] The fish meat spiked with DAP was diluted with a mixed solution of phosphate buffer saline and methanol (10%) in equal volume, and the resulting diluted solution was subjected to the immunochromatographic process.
[0077] The diluted fish meat standard sample was diluted and incubated with labeled antibody and labeled antigen for 11 minutes at 37°C to form immune complexes QB-DAP-mAb-DAP and QB-DAP-mAb-Au@Ag-R-DAP -BSA mixture; after incubation, the solution containing the mixture is added to the glass fiber pad, and through the suction of the water-absorbing pad, it passes through the detection area and the quality control area of the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com