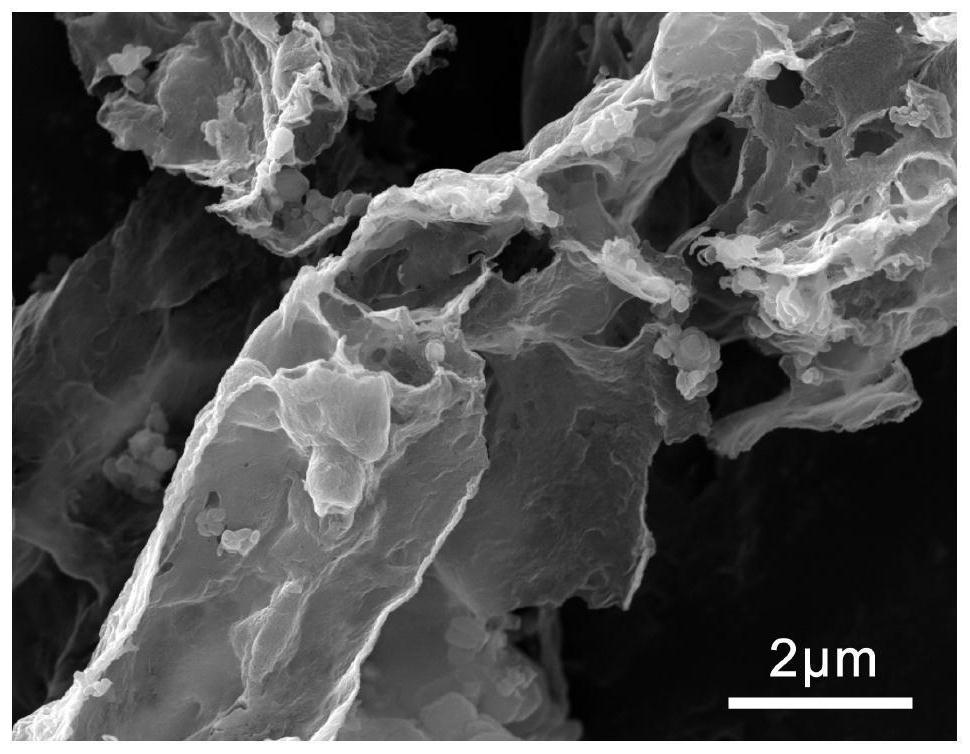
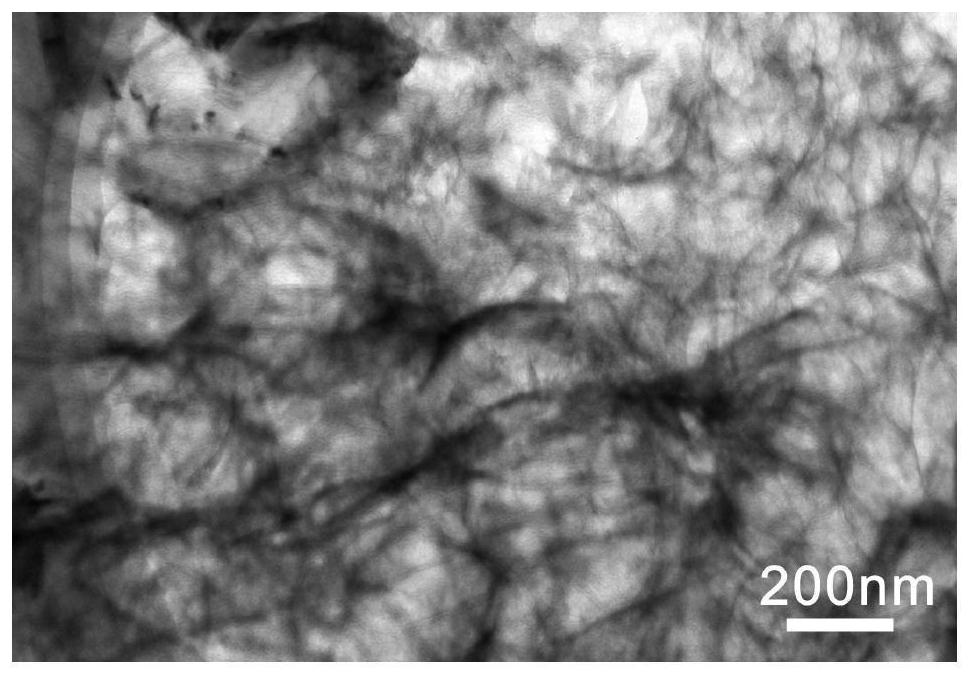
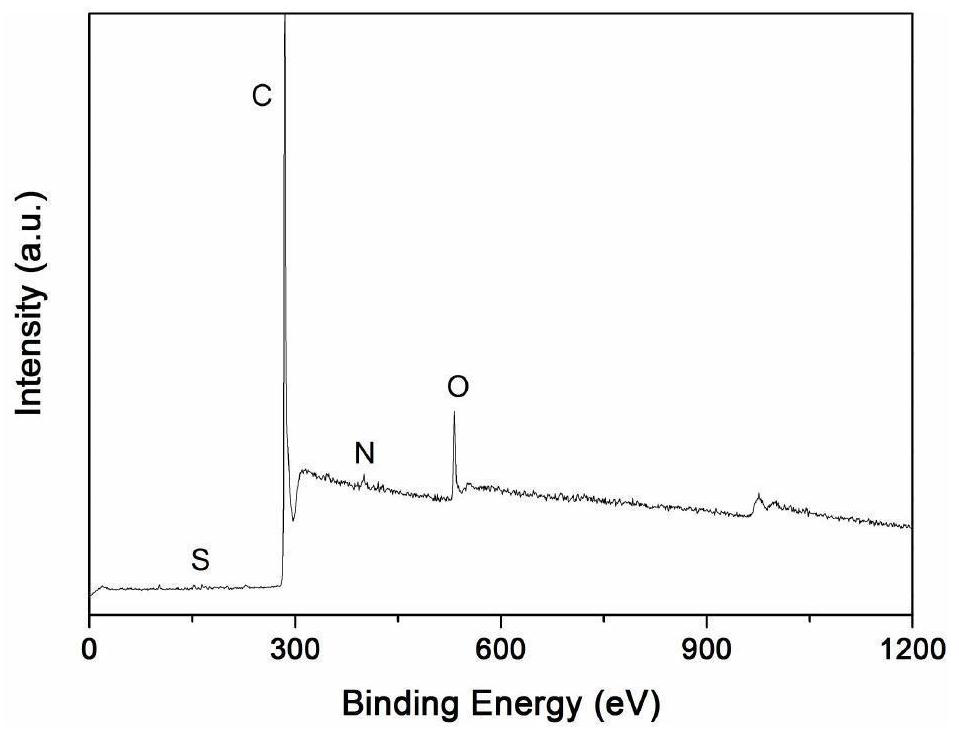
Method for preparing thin-layer graphene negative electrode active material by utilizing antibiotic bacterium dregs
A technology of negative electrode active material and antibiotic slag, which is applied in the field of preparing thin-layer graphene negative electrode active material from antibiotic slag, can solve the problems of complex process, low economic value, and difficulty in treating harmful solid waste of bacterial slag.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Take 20g of dry oxytetracycline slag raw material, first disperse it in 80mL of 20wt.% HF acid solution, stir for 4h, after pumping and filtering, then add it into 80mL of 18wt.%HCl acid solution, stir for 4h, then pass solid Liquid separation, washing to neutrality, suction filtration and drying to obtain dried bacteria residue powder (acid-treated bacteria residue).
[0079] Take 2g of dried bacteria residue powder (acid-treated bacteria residue), add it to 20mL of water, stir for 5min, and add 3g of NaOH, and heat it at 200°C for 12h. After cooling, filter to obtain the liquid part; add 2g Fe(NO 3) 3 .9H 2 O, after stirring for 10 minutes, stir and evaporate to dryness at 100°C, place in a tube furnace filled with nitrogen, first heat at 500°C for 2 hours, continue to heat up to 800°C for 2 hours (heating rate is 5°C / min), cool to room temperature and take out. Pickling, water washing to neutrality and then vacuum drying to obtain a thin-layer graphene negative el...
Embodiment 2
[0082] Compared with Example 1, the difference mainly lies in changing the temperature of the liquefaction process, specifically:
[0083] Take 20g of dry oxytetracycline slag raw material, first disperse it in 80mL of 20wt.% HF acid solution, stir for 4h, after pumping and filtering, then add it into 80mL of 18wt.%HCl acid solution, stir for 4h, then pass solid Liquid separation, washing to neutrality, suction filtration and drying to obtain dried bacteria residue powder (acid-treated bacteria residue).
[0084] Take 2g of dried bacteria residue powder (acid-treated bacteria residue), add it to 20mL of water, stir for 5min, and add 3g of NaOH, and heat it at 120°C for 12h. After cooling, filter to obtain the liquid part; add 2g Fe(NO 3 ) 3 .9H 2 O, after stirring for 10 minutes, stir and evaporate to dryness at 100°C, place in a tube furnace filled with nitrogen, first heat at 500°C for 2 hours, continue to heat up to 500°C for 2 hours (heating rate is 5°C / min), cool to ro...
Embodiment 3
[0087] Compared with Example 1, the difference mainly lies in changing the temperature of the liquefaction process, specifically:
[0088] Take 20g of dry oxytetracycline slag raw material, first disperse it in 80mL of 20wt.% HF acid solution, stir for 4h, after pumping and filtering, then add it into 80mL of 18wt.%HCl acid solution, stir for 4h, then pass solid Liquid separation, washing to neutrality, suction filtration and drying to obtain dried bacteria residue powder (acid-treated bacteria residue).
[0089] Take 2g of dried fungal residue powder (acid-treated fungal residue), add it to 20mL of water, stir for 5min, add 3g of NaOH, and heat at 210°C for 10h. After cooling, filter to obtain the liquid part; add 2g Fe(NO 3 ) 3 .9H 2 O, after stirring for 10 minutes, stir and evaporate to dryness at 100°C, place in a tube furnace filled with nitrogen, first heat at 500°C for 2 hours, continue to heat up to 800°C for 2 hours (heating rate is 5°C / min), cool to room temperat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com