Alkene-functionalized heparin compound and application thereof
A compound and alkene-functional technology, applied in the field of alkene-functionalized heparin compounds, can solve the problems of limited wide application, narrow material range, complicated pretreatment process, etc., and achieve the effects of simple process, firm bonding and wide application range of materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
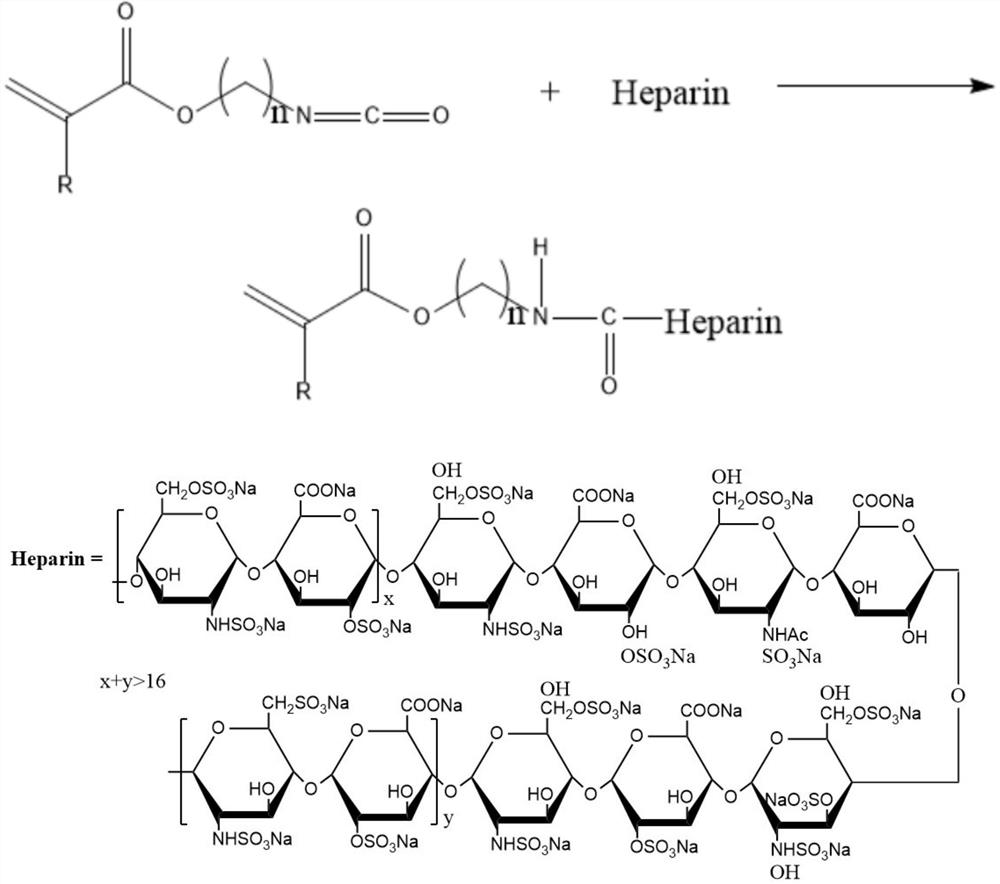
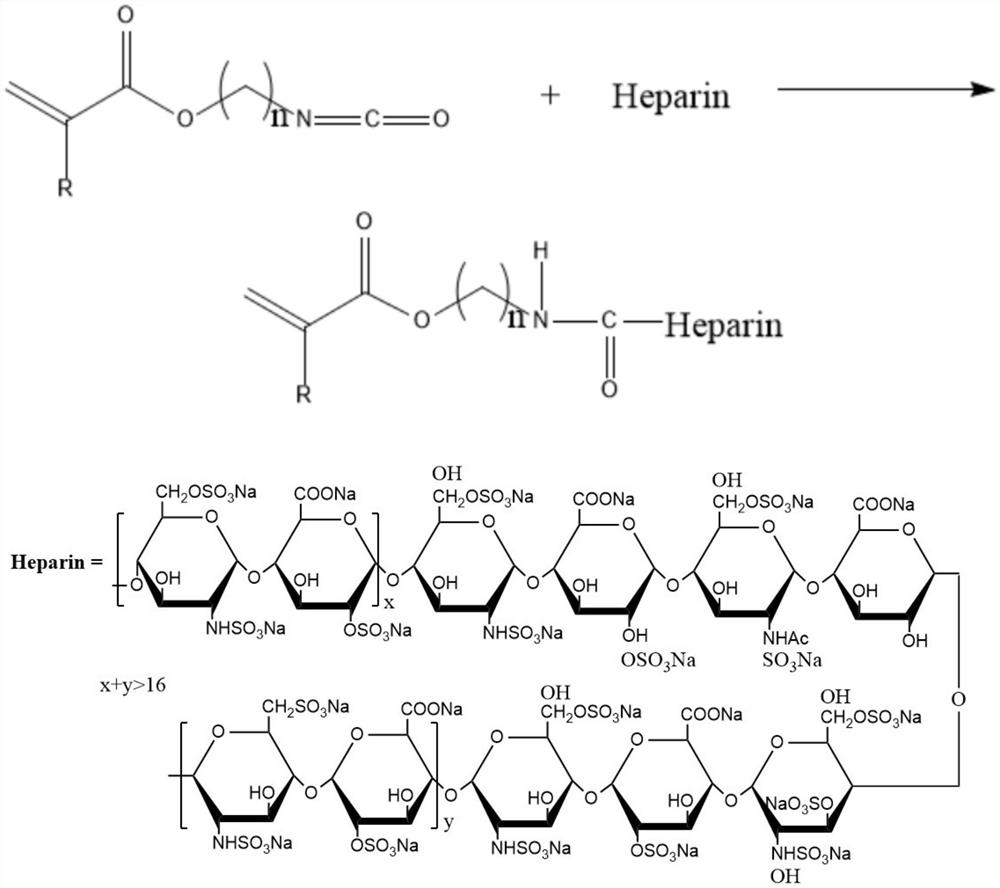
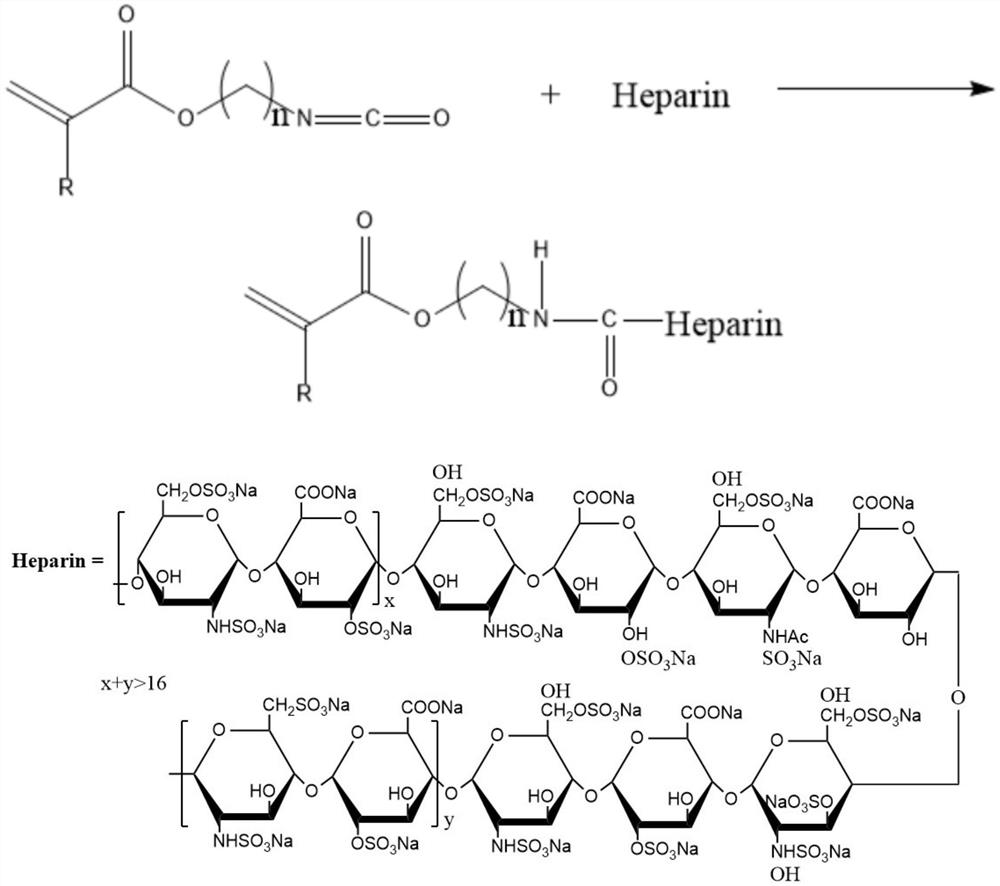
[0027] Preparation of ethylenically functionalized heparin compound: the heparin compound is obtained by reacting heparin with an unsaturated monomer with isocyanate group, the reaction occurs between the isocyanate group of the unsaturated monomer with isocyanate group and the hydroxyl group of the heparin molecule, in order To ensure the complete reaction of heparin molecules, excess isocyanate groups should be made, that is, the molar ratio of isocyanate group unsaturated monomers to heparin is greater than 1:1; the reaction temperature is 50°C-90°C; the reaction time is 4-6 hours. To obtain ene-functionalized heparin, the specific reaction formula is as follows:
[0028]
[0029] Where: R is H or -CH3
[0030] n is an integer between 2-10
[0031] Preparation of the heparin coating solution: the olefin-functionalized heparin compound is mixed with a photoinitiator, a solvent, and a functionalized monomer, oligomer or polymer to prepare a solution, and the olefin-functi...
Embodiment 1-5
[0037] Preparation of ene-functionalized heparin compounds: Dissolve 15g of heparin in 100mL of anhydrous THF, place in a three-necked bottle, stir evenly, and pour into dry N 2 , heated to 60°C; 0.3g of isocyanate ethyl acrylate was dissolved in 20mL of anhydrous THF, placed in a dropping funnel, slowly added dropwise into a three-necked bottle, and the dropwise addition was completed in 30 minutes, and continued to maintain the temperature for 4 hours. Precipitate twice with anhydrous ether and dry in a vacuum oven for 24 hours. An ene-functionalized heparin compound is obtained.
[0038] Preparation of heparin coating solution: In a 200mL brown flask, add ethylenically functionalized heparin, photoinitiator, solvent and functionalized monomer, oligomer or polymer respectively according to the formula shown in Table 1 below, room temperature Stir until the polymer is completely dissolved and a homogeneous solution is formed.
[0039] Table 1 Formulas of different heparin c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com