Recombinant escherichia coli for efficiently producing glutaric acid and construction method of recombinant escherichia coli
A technology for recombining Escherichia coli and Escherichia coli, which is applied in the field of metabolic engineering and can solve problems such as high cost, serious pollution, and high operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Construction of pathway enzyme-related single gene expression vectors
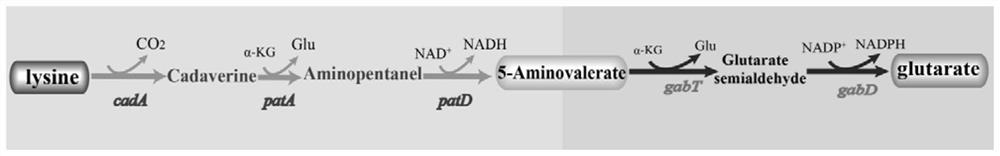
[0028] Lysine decarboxylase cadA (NCBI: NP_418555), butanediamine transaminase patA (NCBI: NP_417544) and gamma-aminobutyraldehyde dehydrogenase patD (NCBI: NP_415961) genes used in the present invention are derived from Escherichia coli MG1655, 4- Aminobutyrate transaminase gabT (NCBI: AEV60208) and succinate semialdehyde dehydrogenase gabD (NCBI: AEV60207) genes are derived from Pseudomonas fluorescens, such as figure 1 shown. Escherichia coli MG1655 and Pseudomonas fluorescens were inoculated in 25mL of LB liquid medium, cultured at 37°C, 200rpm for 10h, the bacteria were collected, and the genomes of Escherichia coli strains and Pseudomonas fluorescens strains were extracted using the bacterial genome extraction kit DNA.
[0029] According to the published genome information sequence, primers corresponding to each pathway enzyme were designed respectively, and the above-mentioned ex...
Embodiment 2
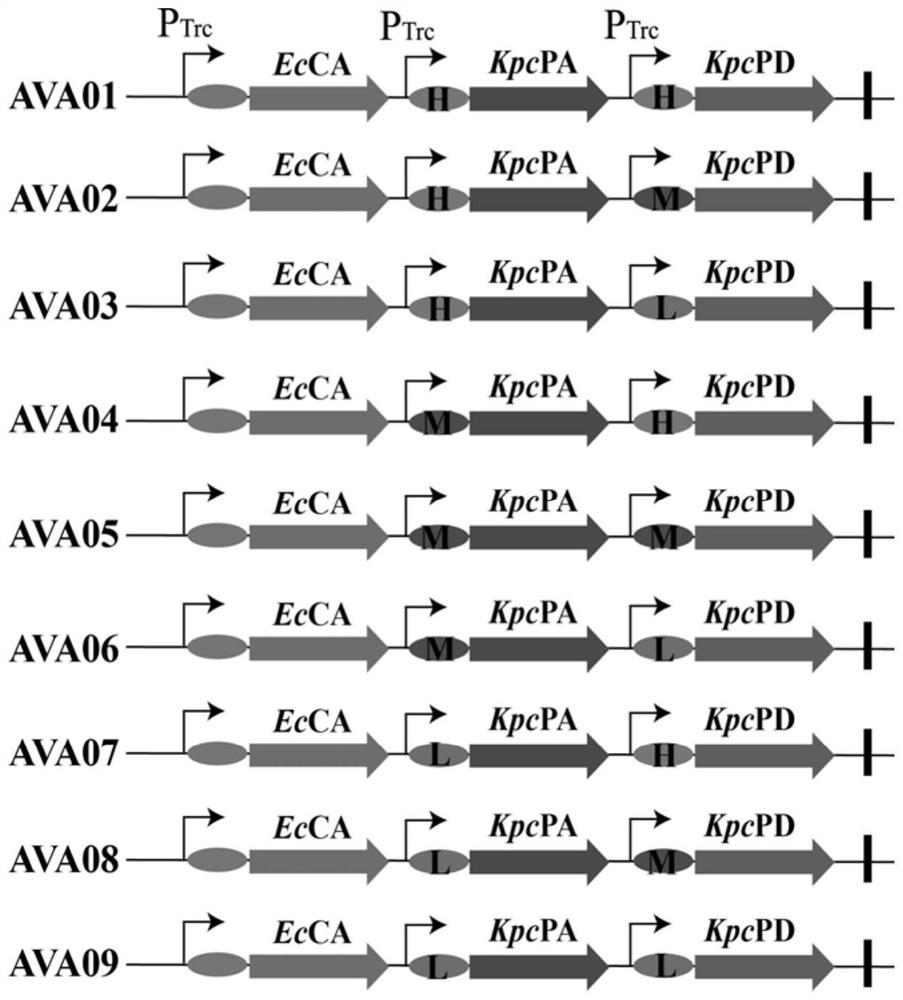
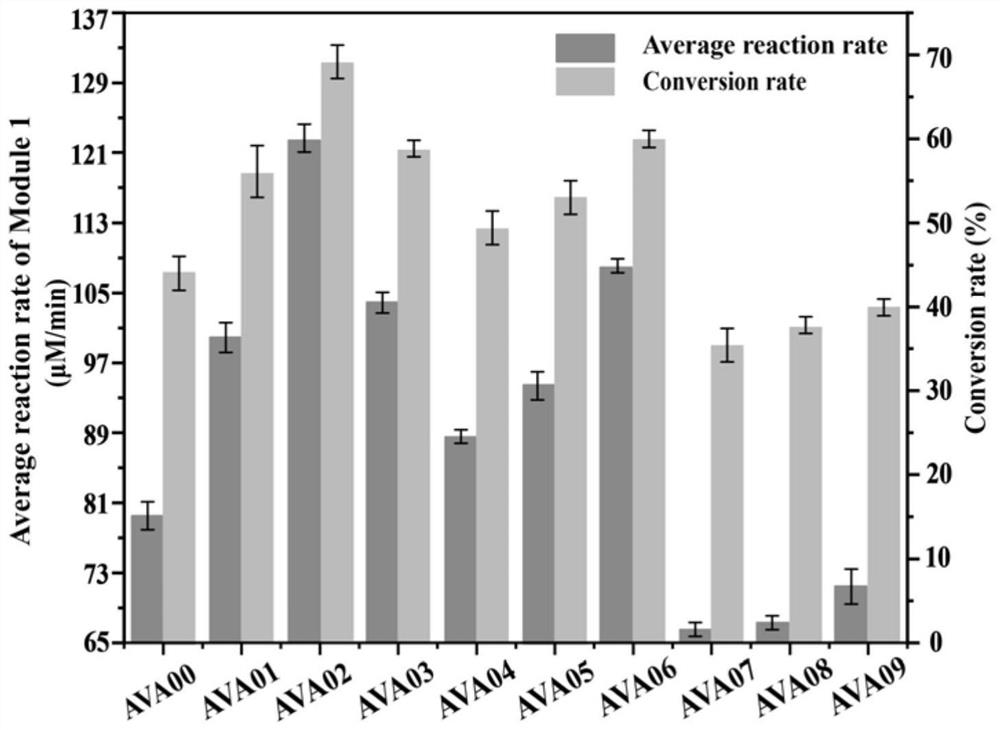
[0030] Example 2: Construction of gene expression vectors with different strengths related to key path enzymes
[0031]The plasmids pETM6R1-patA and pETM6R1-patD obtained in Example 1 were double digested with XbaI and BamHI to obtain a linearized plasmid in which the original RBS sequence on the plasmid pETM6R1 was excised. A total of 3 pairs of amplification primers for RBS01-RBS03 were designed respectively, and the corresponding RBS fragments were amplified using a PCR instrument under the conditions of 95° C. for 5 min. After phosphorylation, the amplified RBS fragment was ligated with the linearized plasmid by T4 DNA ligase. The system was 10 μL: 7.5 μL amplified fragment, 1 μL double restriction vector, 1 μL buffer, 0.5 μL T4DNAligase, ligated overnight at 16°C, and ligated the product Transformed into JM109 competent cells, picked a single colony for PCR verification, and sequenced the positive transformants. The sequencing results were consistent with the theoretical ...
Embodiment 3
[0032] Example 3: Construction of recombinant expression vector (pETM6R1-RBS01 / RBS02 / RBS03-patA-RBS01 / RBS02 / RBS03-patD)
[0033] On the basis of the successfully constructed single-gene expression vectors of different strengths in Example 2, path enzymes were assembled using ePathBrick technology. Taking the construction of the recombinant expression vector pETM6R1-RBS01-patA-RBS02-patD as an example, the steps are introduced. Select the vector pETM6R1-RBS01-patA with restriction endonucleases SpeI and SalI to obtain a linearized vector and expose the cohesive ends; then select the vector pETM6R1-RBS02-patD with AvrII and SalI, and then perform gel recovery. The target gene with cohesive ends was obtained; finally, it was ligated overnight at 16°C with T4 ligase, and the ligated product was transformed into JM109 competent cells, and a single colony was picked for PCR verification. If the band size was correct, it indicated that the recombinant expression vector pETM6R1-RBS01-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com