Pharmaceutical composition as well as preparation method and application thereof
A composition and drug technology, applied in the field of pharmaceutical compositions and their preparation, can solve problems such as unpublished pulmonary degenerative diseases, and achieve the purpose of preventing lung cell apoptosis, inhibiting the secretion of inflammatory cytokines, and promoting the secretion of lung regeneration cytokines. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0104] As the method for preparing CD31 and CD73 positive mesenchymal stem cells of the present invention, the method comprises the following steps:
[0105] Step 1: obtaining and culturing mesenchymal stem cells from the source tissue;
[0106] Step 2: dispersing the mesenchymal stem cells obtained in Step 1 to obtain a single cell suspension;
[0107] Step 3: Sorting and extracting CD31- and CD73-positive mesenchymal stem cells from the single-cell suspension obtained in Step 2.
[0108] These steps are described in detail below.
[0109] (1) Step 1: Obtain and culture mesenchymal stem cells from the source tissue. That is, a collection of mesenchymal stem cells obtained by isolating and culturing mesenchymal stem cells from tissues such as umbilical cord, fat, bone marrow, and placenta, preferably placental tissue, according to methods known to those skilled in the art.
[0110] The growth medium used for culturing mesenchymal stem cells of the present invention is a med...
Embodiment 1
[0199] Example 1: Preparation of CD31 and CD73 positive mesenchymal stem cells
[0200] Placentas were obtained from healthy pregnant women undergoing caesarean section, and informed consent statements were signed according to ethical requirements. After the placenta was collected, the decidua membrane and umbilical cord were peeled off, and the cotyledons were preserved. The cotyledons were then washed in Hanks buffered saline solution (HBSS) and then digested in a solution of 1 mg / mL collagenase I, 1 mg / mL Dnase-1 and 75 μg / mL Dispase at 37°C for 2 hours. After digestion, filter the single cell suspension through a 100 µm sieve and spin at 750 x g for 5 min. The supernatant was decanted and the cell pellet was then resuspended in erythrocyte lysis buffer and incubated in a 37°C incubator for 10 minutes. The suspension was then spun at 510 x g for 5 h min. The supernatant was then decanted, and the cell pellet was washed in Hanks buffered saline solution (HBSS) and spun ag...
Embodiment 2
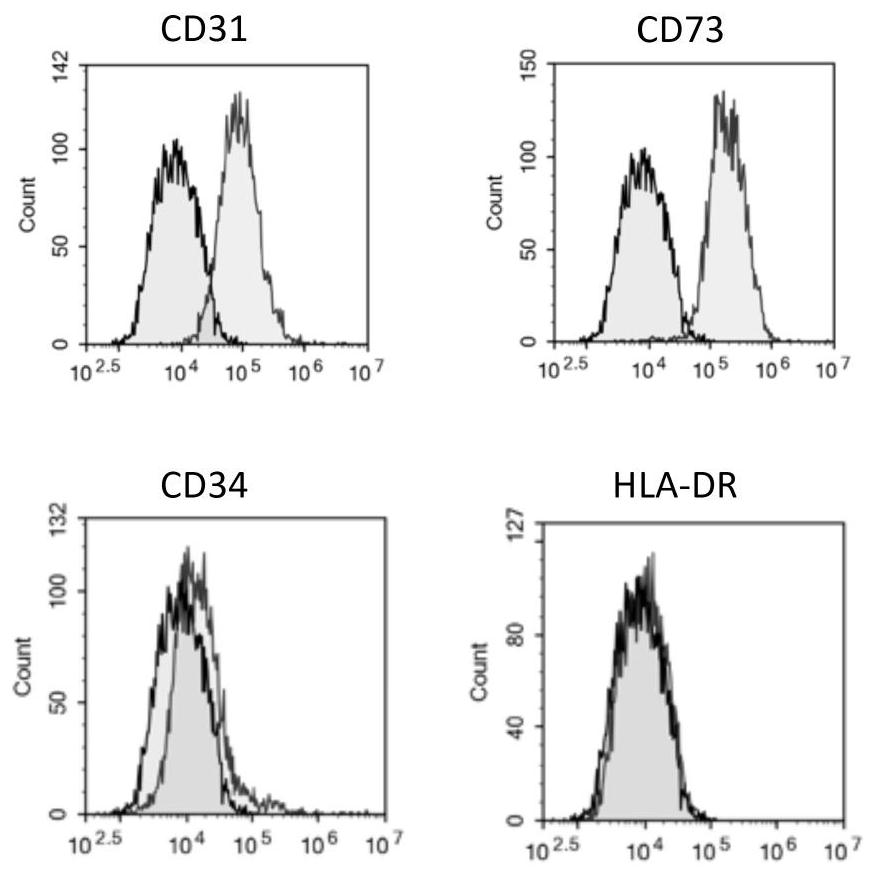
[0203] Example 2: Detection of CD31 and CD73 positive mesenchymal stem cell surface markers
[0204]The third-generation mesenchymal stem cells cultured in Example 1 were taken, and the expressions of CD31, CD73, CD34 and HLA-DR were detected by flow cytometry. Reagents used are BD Pharmingen, USA, respectively FITC mouse anti-human CD31 (Cat#560984), PE mouse anti-human CD73 (Cat#550257, BD), FITC mouse anti-human CD34 (Cat#555821), FITC mouse anti-human HLA-DR (Cat#555811). Control antibodies are FICT mouseIgG1, k isotype (Cat#555909), PE mouse IgG2b, k isotype control (Cat#559529).
[0205] Staining and flow detection were carried out according to the experimental method provided by BD Pharmingen in the United States, and the results are shown in figure 2 .
[0206] The results showed that: the mesenchymal stem cells cultured in Example 1 were positive for expression of CD31 and CD73, and negative for expression of CD34\CD45 and HLA-DR.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com