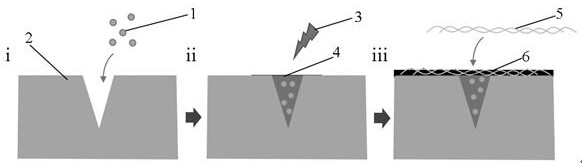
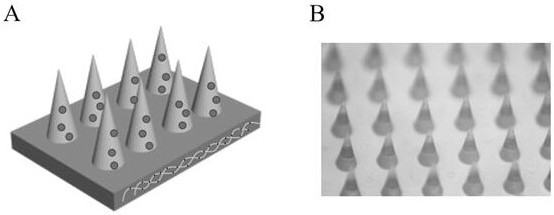
Preparation method and application of antibacterial damage repair microneedle for slowly releasing MSC (mesenchymal stem cell) sourced exosome
A technology of damage repair and exosomes, which is applied in the direction of microneedles, antibacterial drugs, and devices introduced into the body, can solve the problems of unstable treatment effect and large infusion dose, and improve the oxidative and inflammatory environment of the wound, blood medicine The effect of stable concentration and reduced frequency of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of antimicrobial repairing microneedles with slow-release MSC-exo, the steps are as follows:
[0040] (1) Preparation of gelatin methacrylate (GelMA) aqueous solution:
[0041] Weigh GelMA, add to double distilled water to disperse, prepare 30% GelMA solution, fully dissolve;
[0042] (2) Isolation of exosomes derived from mesenchymal stem cells:
[0043] Select MSCs in the logarithmic growth phase for the isolation and extraction of exosomes, and use MSC complete medium for culture. When the cells grow to 80% confluence, replace the MSC complete medium with serum-free exosome medium , continue culturing for 48 hours; collect the cell culture supernatant, and extract exosomes by ultracentrifugation. The ultracentrifugation conditions are: 50,000 rpm, 4°C, 60 min;
[0044] (3) Preparation of microneedle units loaded with MSC exosomes:
[0045] The exosomes derived from the 4th generation MSC were uniformly mixed at 50 μg / ml in the GelMA solution contai...
Embodiment 2
[0049] The preparation of antimicrobial repairing microneedles with slow-release MSC-exo, the steps are as follows:
[0050] (1) Preparation of methacrylate hyaluronic acid aqueous solution:
[0051] Weigh a certain amount of methacrylate hyaluronic acid, add it to double-distilled water to disperse, prepare a 40% aqueous solution, and fully dissolve it;
[0052] (2) Isolation of exosomes derived from mesenchymal stem cells:
[0053] Select MSCs in the logarithmic growth phase for the isolation and extraction of exosomes, and use MSC complete medium for culture. When the cells grow to 80% confluence, replace the MSC complete medium with serum-free exosome medium , continue culturing for 48 hours; collect the cell culture supernatant, and extract exosomes by ultracentrifugation. The ultracentrifugation conditions are: 60,000 rpm, 4°C, 120 min;
[0054] (3) Preparation of microneedle units loaded with MSC exosomes:
[0055] The exosomes derived from the third-generation MSCs ...
Embodiment 3
[0059] The preparation of antimicrobial repairing microneedles with slow-release MSC-exo, the steps are as follows:
[0060] (1) Preparation of gelatin methacrylate (GelMA) aqueous solution:
[0061] Weigh GelMA, add to double distilled water to disperse, prepare 35% GelMA solution, fully dissolve;
[0062] (2) Isolation of exosomes derived from mesenchymal stem cells:
[0063] Select MSCs in the logarithmic growth phase for the isolation and extraction of exosomes, and use MSC complete medium for culture. When the cells grow to 80% confluence, replace the MSC complete medium with serum-free exosome medium , continue culturing for 48 hours; collect the cell culture supernatant, and extract exosomes by ultracentrifugation. The ultracentrifugation conditions are: 65,000 rpm, 4°C, 60 min;
[0064] (3) Preparation of microneedle units loaded with MSC exosomes:
[0065] The exosomes derived from the 4th generation MSC were evenly mixed in the GelMA solution containing 1% (v / v) p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com