PH response type anti-tumor prodrug by using polyethylene glycol-b-poly-epsilon-caprolactone as vector and preparation method thereof
A polyethylene glycol and anti-tumor drug technology, applied in the field of biomedicine, can solve the problems of low drug bioavailability, high drug loading capacity of polymer carriers, good biocompatibility, etc. Release, good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] 1. Preparation of α-bromo ε-caprolactone monomer
[0073] Weigh 38.14g of bromosuccinimide (NBS), 20.00g of cyclohexanone, 1.54g of ammonium acetate, and 300mL of anhydrous ether into a 1000mL round bottom flask, add a magnet to stir, and react at room temperature for 1h. After the reaction is completed, filter, wash with distilled water three times, and separate through a column to obtain the product α-position bromocyclohexanone. Weigh 17.70 g of the obtained α-position bromocyclohexanone and 20.64 g of m-chloroperoxybenzoic acid (m-CPBA) and dissolve them in a 500 mL single-necked flask with 300 mL of dichloromethane, stir and react at room temperature for 48 h; filter after the reaction, and the filtrate Place in the refrigerator overnight and then filter out the precipitated m-chlorobenzoic acid. The filtrate was washed three times with saturated sodium thiosulfate, sodium bicarbonate and distilled water respectively. After the organic phase was dried and concentra...
Embodiment 2
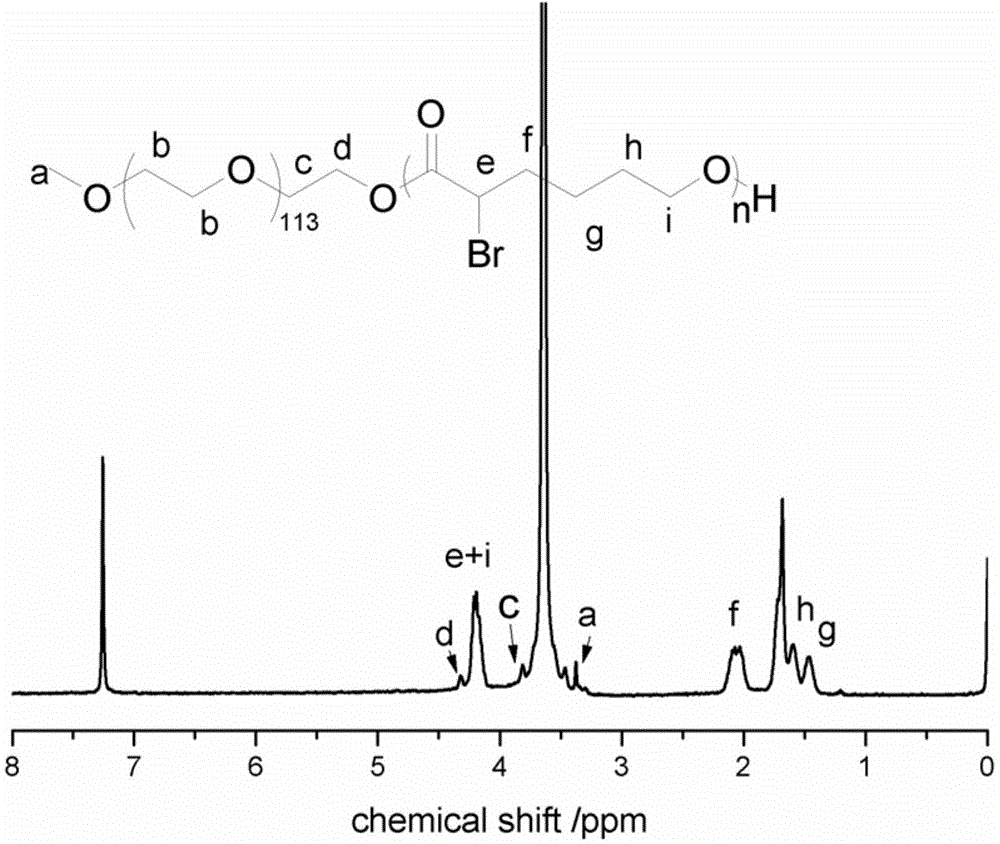
[0081] Steps 1 and 2 The method for synthesizing polyethylene glycol-b-poly α-position brominated ε-caprolactone amphiphilic block copolymer is the same as that of Example 1.
[0082] 3. Functionalization of antitumor drug doxorubicin
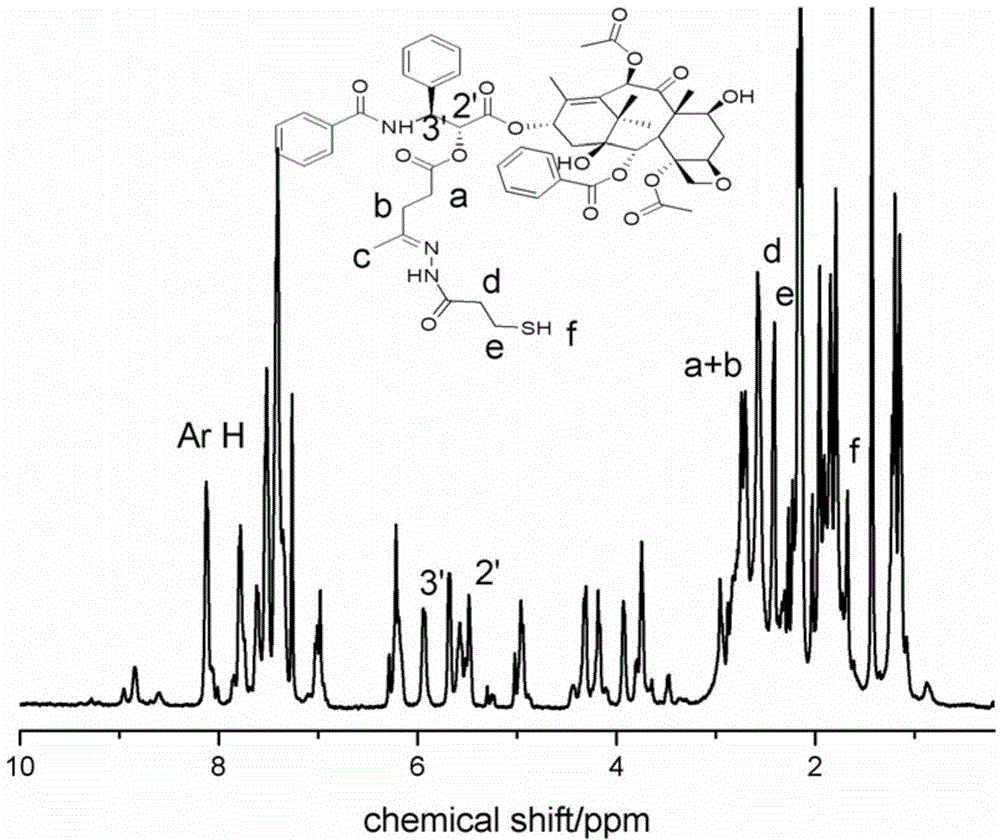
[0083] Weigh 116mg of doxorubicin hydrochloride and dissolve it in 50mL of anhydrous methanol, dissolve 48mg of 3-mercaptopropionylhydrazide in 2mL of anhydrous methanol, add it dropwise into the reaction bottle under the protection of argon, and react for 24 hours at room temperature in the dark, and the resulting functionalized Doxorubicin was settled with anhydrous ether, and centrifuged to obtain a dark red solid (NMR see Image 6 ).
[0084] 4. Preparation of pH-responsive doxorubicin prodrug
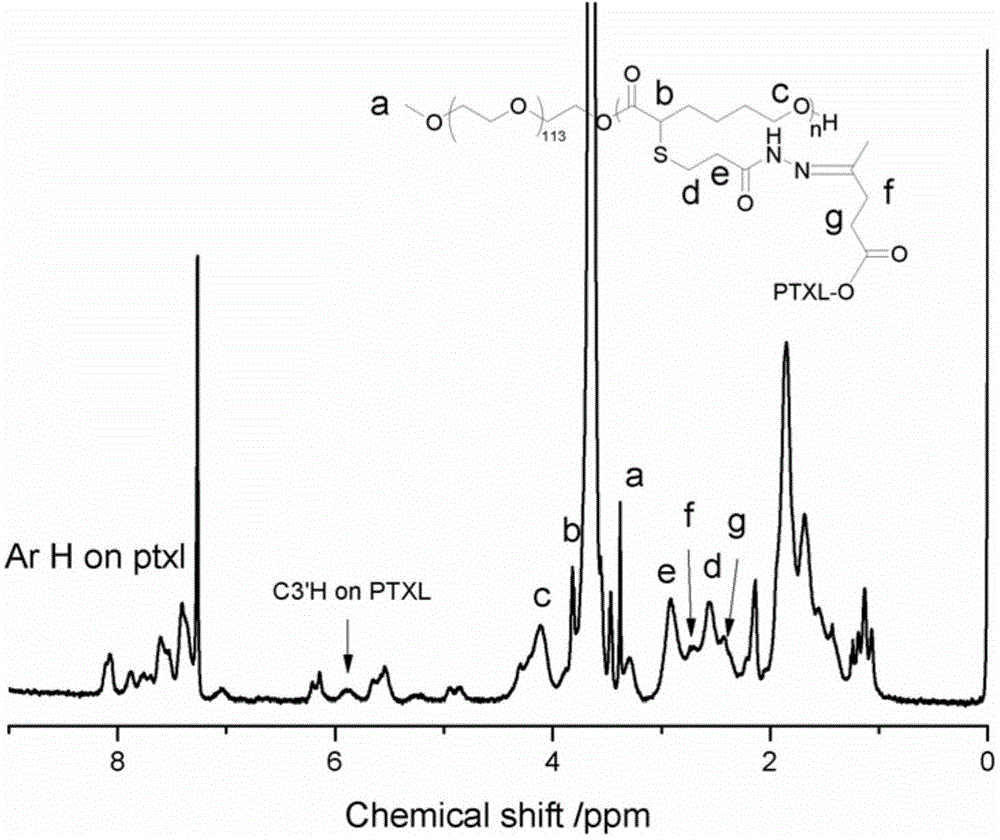
[0085] Weigh 75 mg of polyethylene glycol-b-polyα-bromoε-caprolactone amphiphilic block copolymer, 75 mg of functionalized doxorubicin, dissolve in 5 mL of anhydrous DMF, add triethylene glycol dropwise under argon protection Amine 12mg, reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com