Preparation method of lithium bis (fluorosulfonyl) imide
The technology of lithium bisfluorosulfonimide and bisfluorosulfonimide is applied in the field of preparation of lithium bisfluorosulfonimide, which can solve the problems of low extraction efficiency, difficult separation and high energy consumption, and shorten the reaction time. , The effect of less environmental pollution and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032]The specific implementation of the present invention will be further described below in conjunction with examples. The following embodiments are only used to illustrate the technical solutions of the present invention more clearly, and cannot be used to limit the protection scope of the present invention.
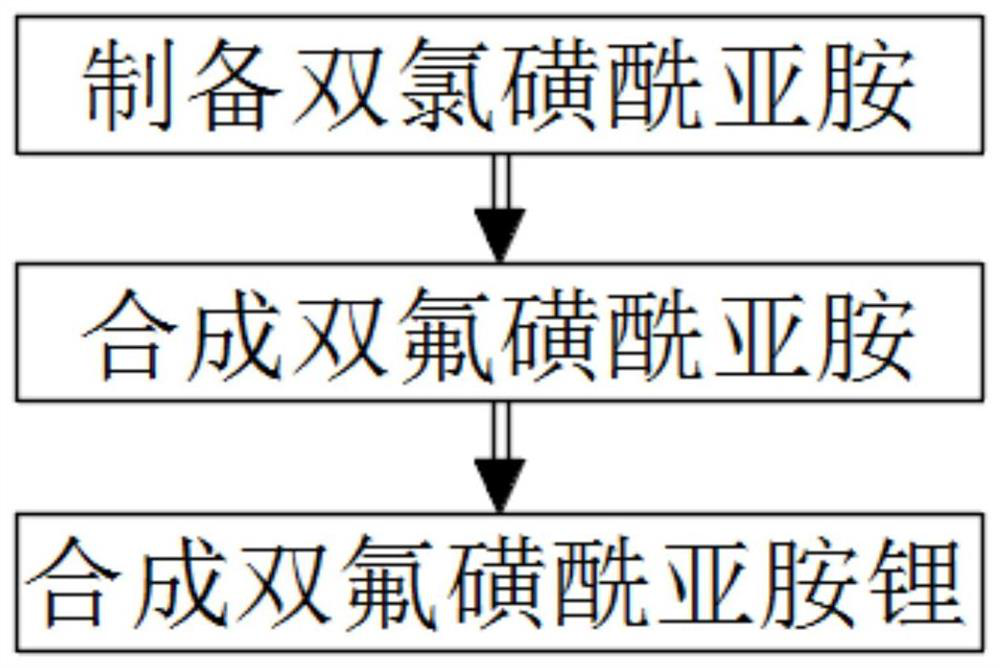
[0033]Such asfigure 1As shown, a method for preparing lithium bisfluorosulfonimide, the specific steps of the method are as follows:
[0034]Step 1. Preparation of bischlorosulfonimide;
[0035]Step 2: Synthesis of bisfluorosulfonimide;
[0036]Step 3: Synthesis of lithium bisfluorosulfonimide.
[0037]The preparation of bischlorosulfonimide in step one includes:
[0038]1) Using chlorosulfonyl isocyanate and chlorosulfonic acid as the reaction raw materials, the reaction was carried out dropwise at 80-100°C for 5 hours, and then heated to 110-120°C for 10 hours incubation to prepare dichlorosulfonimide Crude;
[0039]2) The crude bischlorosulfonimide is rectified, and bischlorosulfonimide with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com