Synthetic method of Semaglutide
A synthesis method and peptide sequence technology, applied in the field of synthesis of GLP-1 analogue semaglutide, can solve problems such as poor coupling effect, achieve improved coupling effect, avoid peptide chain aggregation, improve purity and The effect of synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
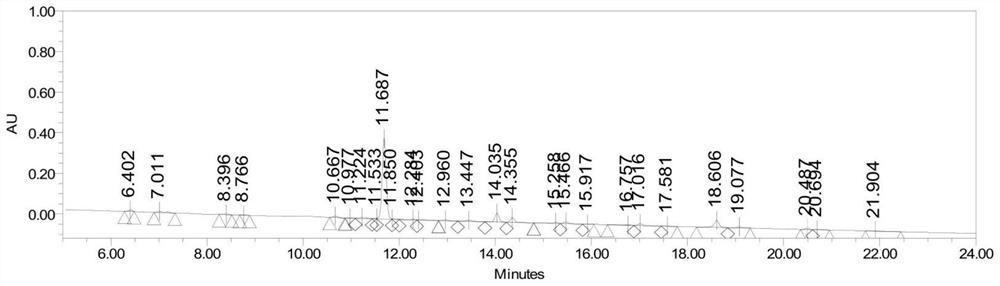
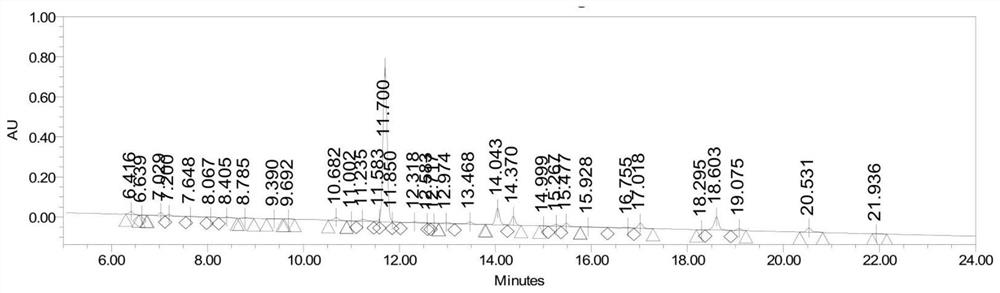
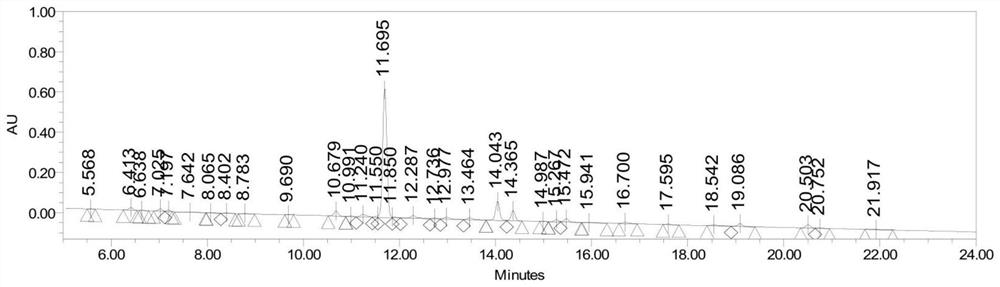
Image
Examples
preparation example Construction
[0040] The invention discloses a preparation method of semaglutide, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
[0041] The specific meanings of the abbreviations used in the specification and claims are as follows:
[0042] Abbreviation and English meaning Alloc Allyloxycarbonyl DIC Diis...
Embodiment 1
[0045] The preparation of embodiment 1 Merrifield Resin and imidazole ethanol solid phase carrier
[0046] Take by weighing 120mmol of imidazole ethanol, dissolve in THF, add dropwise an appropriate amount of concentrated sulfuric acid, add 180mmol of liquid isobutene under ice-bath conditions, stir overnight at room temperature, crystallize after extraction, and obtain imidazole ethanol (compound 1 ).
[0047] Weigh 125 grams of Merrifield Resin (100mmol, 0.8mmol / g), add the previously obtained compound 1, react in N-methylpyrrolidone at 80°C for 24 hours, filter with suction, wash the solid phase resin with DCM, and then add 25% TFA in DCM solvent was reacted at room temperature for 2 hours to obtain tert-butyl-protected imidazole ethanol Merrifield resin.
[0048] The imidazole ethanol Merrifield resin protected by tert-butyl group is divided into four parts, respectively with sodium tetrafluoroborate, sodium hexafluorophosphate, sodium trifluoromethanesulfonate, lithium b...
Embodiment 2
[0050] The preparation of embodiment 2 Merrifield Resin and imidazole propanol solid phase carrier
[0051] With reference to the synthetic method of Example 1, react with imidazole propanol and Merrifield Resin to make Merrifield Resin with imidazole propanol ionic liquid as Linker, the specific structure is as follows:
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com