Feline calicivirus VP1-VP2 recombinant protein as well as preparation method and application thereof
A VP1-VP2, feline calicivirus technology, applied in biochemical equipment and methods, viruses, viral peptides, etc., has achieved great clinical significance, wide application prospects, and improved sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1 Contains the construction of feline calicivirus VP1-VP2 fusion protein gene expression vector
[0062] Feline calicivirus VP1 gene was designed according to the protein sequence of NCBI Gene bank: P27406.1. The VP2 gene was designed according to the protein sequence of NCBI Gene bank: ALM55430.
[0063] The amino acid sequence of the VP1-VP2 recombinant protein is as follows (SEQ ID NO.1):
[0064] ADDGSITAPEQGTMVGGVIAEPSAQMSTAADMATGKSVDSEWEAFFSFHTSVNWSTSETQGKILFKQSLGPLLNPYLEHLAKLYVAWSGSIEVRFSISGSGVFGGKLAAIVVPPGVDPVQSTSMLQYPHVLFDARQVEPVIFCLPDLRSTLYHLMSDTDTTSLVIMVYNDLINPYANDANSSGCIVTVETKPGPDFKFHLLKPPGSMLTHGSIPSDLIPKTSSLWIGNRYWSDITDFVIRPFVFQANRHFDFNQETAGWSTPRFRPISVTITEQNGAKLGIGVATDYIVPGIPDGWPDTTIPGELIPAGDYAITNGTGNDITTATGYDTADIIKNNTNFRGMYICGSLQRAWGDKKISNTAFITTATLDGDNNNKINPCNTIDQSKIVVFQDNHVGKKAQTSDDTLALLGYTGIGEQAIGSDRDRVVRISTLPETGARGGNHPIFYKNSIKLGYVIRSIDVFNSQILHTSRQLSLNHYLLPPDSFAVYRIIDSNGSWFDIGIDSDGFSFVGVSGFGKLEFPLSASYMGIQLAKIRLASNIRSPMTKLMNSILGLIDTVTNTIGKAQQIE...
Embodiment 2
[0070] Embodiment 2 contains the expression of feline calicivirus VP1-VP2 fusion protein
[0071]Transform the synthetic feline calicivirus VP1-VP2 fusion gene plasmid into Escherichia coli BL21, smear it on an LB plate containing 50 μg / mL kanamycin (Shanghai Sangong, product number: K0408), and culture overnight at 37°C , picked a single clone colony, cultured in 300mL LB medium containing the same concentration of kanamycin at 37°C until the OD600 reached about 0.6, and induced expression with IPTG (Shanghai Sangong, product number: IB0168) with a final concentration of 1mM, The induction conditions are: 25°C, 200rpm rotation speed, 4h. After induction, the culture solution was centrifuged at 4° C. at 7000 rpm for 10 min to collect the bacteria.
Embodiment 3
[0072] Embodiment 3 Purification and renaturation containing feline calicivirus VP1-VP2 fusion protein
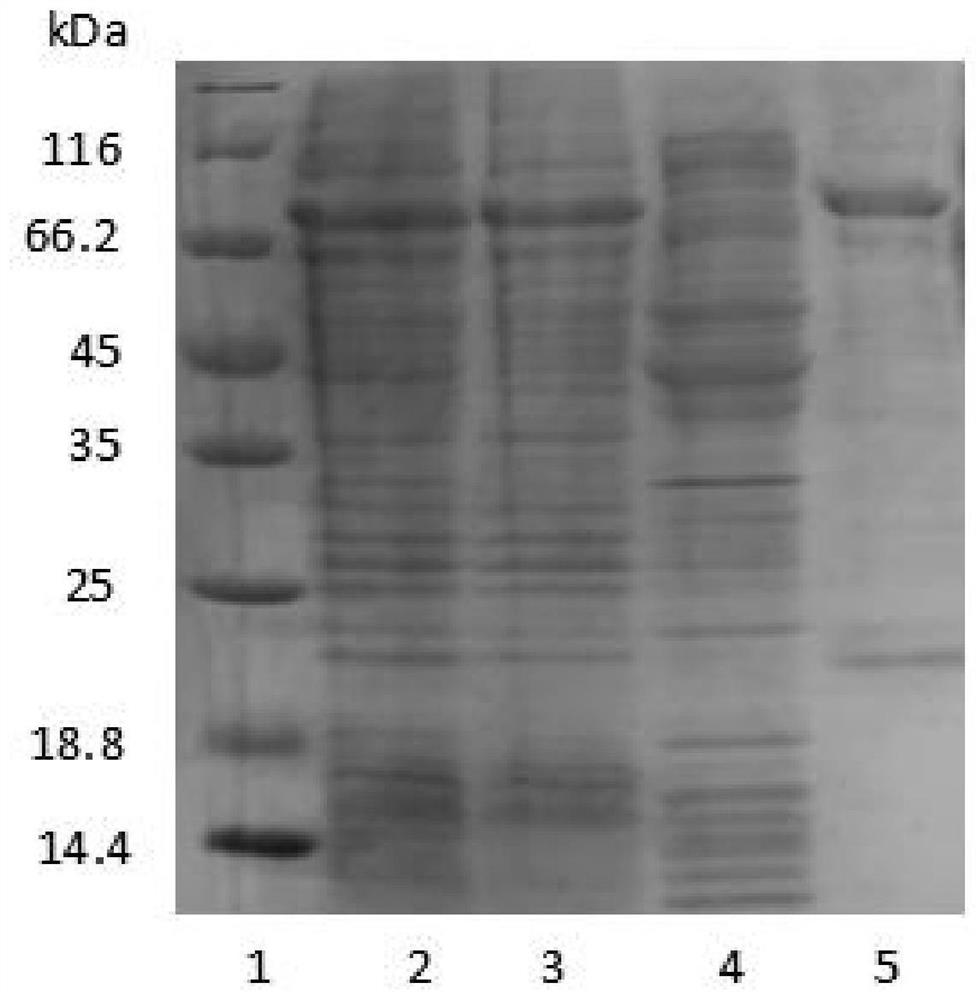
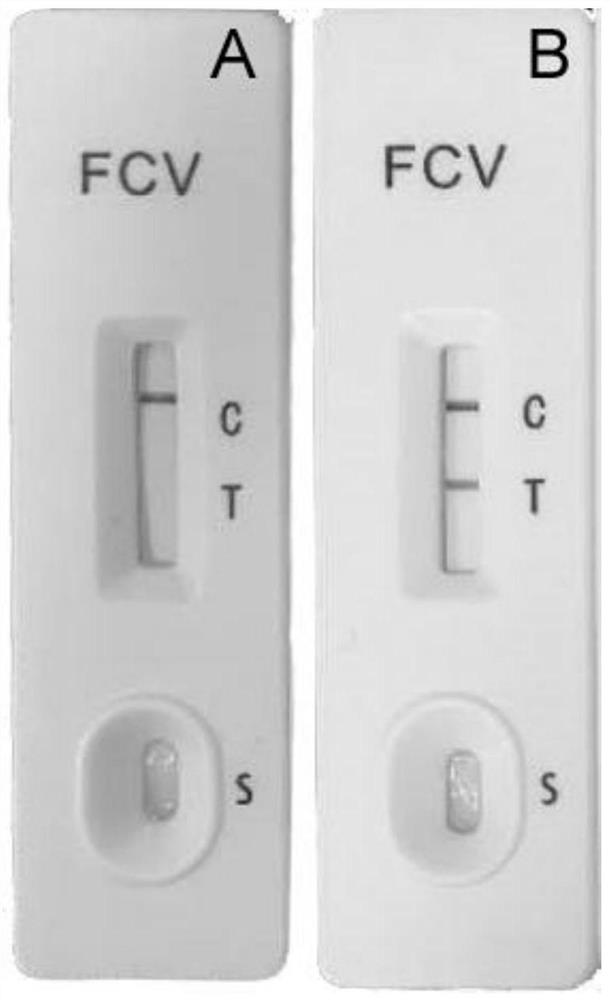
[0073] Use 50mL of loading buffer Binding Buffer (50mM Tris, 0.2M Nacl, pH8.0) 50mL to break up the bacteria; then ultrasonically break, the condition is 500w, ultrasonic 2s, interval 5s, a total of 100 times; finally 12000rpm, 30min, 4℃ Collect the supernatant by centrifugation, and the target protein is in the supernatant. Then, it was purified by Ni column, and the target protein was eluted with Elution Buffer (50mM Tris, 0.2M Nacl, 0.5M Imidazole, pH8.0). The target protein was detected by PAGE gel electrophoresis, and the results were as follows figure 1 shown.
[0074] Depend on figure 1 It can be seen that the purified fusion protein has a high purity. The purified recombinant protein was dialyzed with a dialysis buffer (50mM Tris, 0.2M Nacl, pH8.0), and the dialysis solution was changed every 12 hours for a total of 3 times. The dialyzed protein solution was tak...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com