Triphenylphosphine modification-based mitochondrion targeted melatonin as well as preparation method and application thereof
A technology of triphenylphosphine and mitochondria, which is applied in the field of mitochondria-targeted melatonin modified by triphenylphosphine and its preparation, can solve the problems that melatonin does not have mitochondrial targeting, and achieve the prevention and treatment of mitochondria-related diseases Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
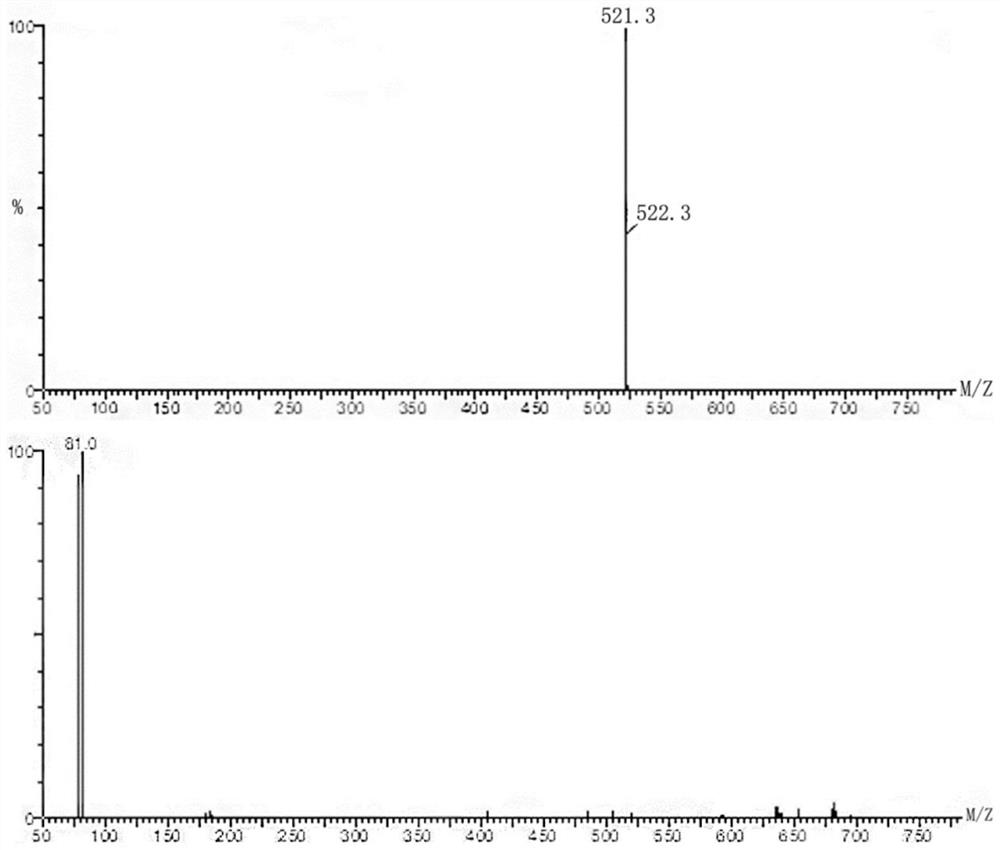
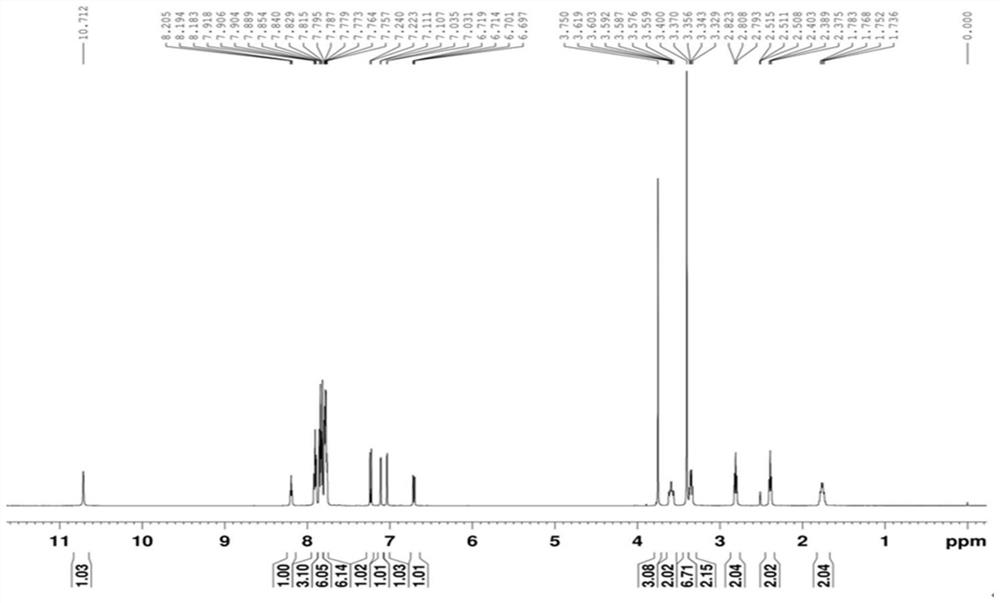
Image
Examples
Embodiment 1
[0051] Embodiment 1: the preparation of the compound represented by formula (IV)
[0052] In this example, the compound represented by formula (IV) is specifically brominated (4-((2-(5-methoxy-1H-indol-3-yl)ethyl)amino)-4-oxo Butyl)triphenylphosphine, see formula (VII) for the specific compound structure.
[0053] The synthetic process of formula (VII) is roughly as follows: bromide (3-carboxypropyl) triphenylphosphine is obtained by reacting tetrabromobutyric acid and triphenylphosphine in an aprotic solvent, after removing the solvent, in an aprotic solvent After reacting with carbon-based diimidazole, then reacting with 5-methoxytryptamine to obtain the product, distilling off the solvent and recrystallizing with distilled water to obtain brominated (4-((2-(5-methoxy-1H-indole- 3-yl)ethyl)amino)-4-oxobutyl)triphenylphosphine.
[0054] The synthesis process of formula (VII) is specifically: 16.7 grams of 4-bromobutyric acid, 26.3 grams of triphenylphosphine, and 50 millili...
experiment example 1
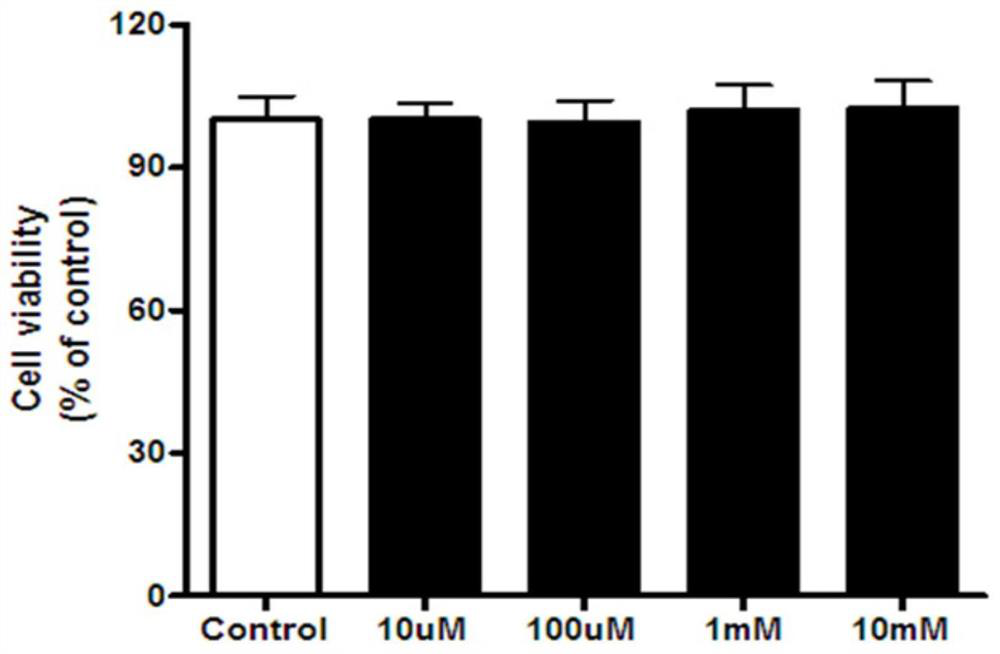
[0070] Experimental Example 1: Toxicity Study of Compounds
[0071] After treated with different concentrations of Mito-Mel (10uM-10mM) for 24 hours, the cell viability of Neuro-2a cells (neuroma blastocytes derived from mice) was not changed compared with the control group. Experimental results such as image 3 As shown, the data format in the figure is: Mean±SEM, N=5. The specific detection method of this experimental example is as follows: the cell viability was detected with a CCK-8 kit. CCK-8 is a rapid and high-sensitivity detection reagent widely used in cell proliferation and cytotoxicity based on WST-8. In the experiment, the 5×10 4 Cells were seeded in a 96-well plate with a total volume of 100 μl per well. When detecting cell activity, aspirate the medium in each well, and add 100ul of fresh medium containing CCK-8 (the volume ratio of CCK-8 to medium is 1:10), and incubate at 37°C for 2h. Then use a microplate reader to measure the OD value of each well, selec...
experiment example 2
[0072] Experimental Example 2: The protective effect of the compound on the cytotoxicity of heavy metal cadmium
[0073] Experimental results such as Figure 4 As shown, Mito-Mel (1mM) can effectively improve heavy metal cadmium exposure (CdCl 2) caused cell damage. Moreover, under the same conditions, the protective effect of Mito-Mel on cell viability after 24h exposure to cadmium was better than that of non-targeted melatonin itself. For the specific detection method, see Experimental Example 1 (CCK-8 method), in Figure 4 Among them, the data form is Mean±SEM, N=5; * indicates that compared with the control group, p2 p<0.05 compared to treatment group.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com