Method for nitration synthesis of acifluorfen in microreactor
A technology of acifluorfen and microreactors, which is applied in the preparation of nitro compounds, chemical instruments and methods, chemical/physical/physical chemical reactors, etc., can solve the harsh and safe synthesis conditions of acifluorfen Low problems, to achieve the effect of fast mass transfer rate and reaction rate, increased heat transfer efficiency, and easy to scale up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
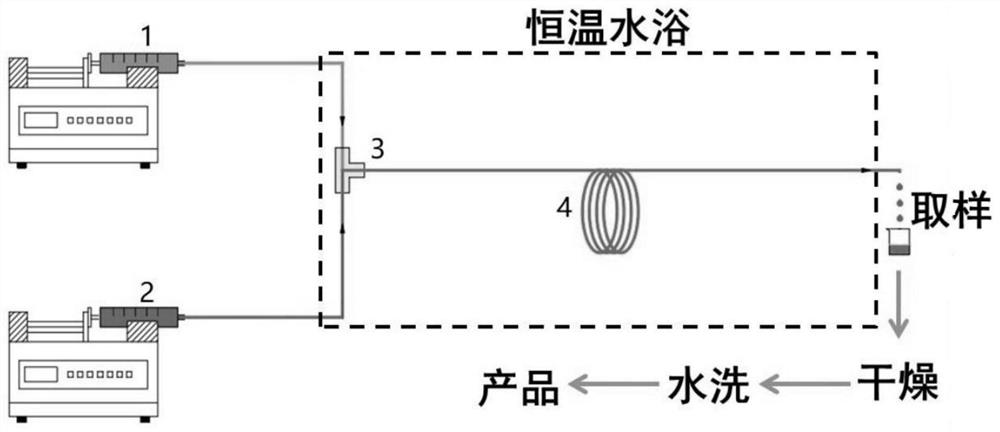
[0028] The mixed acid was prepared by using 98 wt% fuming nitric acid and 20 wt% fuming sulfuric acid according to the nitric acid:sulfuric acid molar ratio of 0.55:1, and the temperature of the mixed acid preparation process was controlled at 15-20 °C. The mass fractions of 3-(2-chloro-4-trifluoromethylphenoxy)benzoic acid, acetic anhydride, and dichloroethane in the organic phase were 15.8%, 5.3%, and 78.9%, respectively.
[0029] At 15 °C, the mixed acid and organic phase were pumped into the micro-mixer by a high-pressure syringe pump through the pipeline. The micro-mixer adopted a T-shaped mixing method and passed into a microchannel reactor with a diameter of 500 μm; nitric acid and 3-(2 The molar ratio of -chloro-4-trifluoromethylphenoxy)benzoic acid is 1.4:1, the reaction residence time is 540 s, the reaction product flows out of the reactor, and the product is obtained by phase separation, drying and washing, and the reaction conversion rate reaches 99.19 %, selectivi...
Embodiment 2
[0043]The process is the same as in Example 1, the molar ratio of mixed acid nitric acid: sulfuric acid remains unchanged, and the mass fraction of 3-(2-chloro-4-trifluoromethylphenoxy)benzoic acid in the organic phase is 8%. At 10 °C, the molar ratio of nitric acid to 3-(2-chloro-4-trifluoromethylphenoxy)benzoic acid was 0.96:1, the reaction residence time was 30 s, the reaction conversion was 53.13%, and the selectivity was 80.32 %.
Embodiment 3
[0045] The process is the same as in Example 1, the molar ratio of mixed acid nitric acid: sulfuric acid remains unchanged, and the mass fraction of 3-(2-chloro-4-trifluoromethylphenoxy)benzoic acid in the organic phase is 30%. At 35 °C, the molar ratio of nitric acid to 3-(2-chloro-4-trifluoromethylphenoxy)benzoic acid was 1.8:1, the reaction residence time was 780 s, the reaction conversion rate was 99.98%, and the selectivity was 69.21 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com