Application of diosmetin(4-O-methyl) glucoside compound in preparation of lipid-lowering drugs
A technology of diosmin and glucoside, which is applied in the fields of drug combination, sugar derivatives, organic chemistry, etc., and can solve the problem of few reports on lipid-lowering activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Methyl glycosylation modification of diosmin by Isaria fumosorosea ACCC 37814
[0016] 1.1 Instruments and materials
[0017] The strain used in the experiment: Isaria fumosorosea ACCC 37814 was obtained from the China Agricultural Microorganism Culture Collection Center (ACCC). Compounds: purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). The reagents used in the experiment were domestic analytically pure products. Culture medium: the fungal medium is potato dextrose agar (PDA, Difco, Sparks, MD, USA) medium and potato dextrose broth (PDB, Difco, Sparks, MD, USA) medium, if making solid medium, add 2 % agar powder.
[0018] High performance liquid chromatography (Agilent, Agilent 1260Infinity II HPLC and Agilent1290Infinity II HPLC), C 18 Chromatographic columns (Agilent, Poroshell 120SB-C18, 2.7 μm, 4.6 mm×150 mm and Zorbax SB-C18, 1.8 μm, 2.1×50 mm). Mass spectrometry (Agilent, Agilent QTOF 6530). Rotary evaporator (BUCHI compan...
Embodiment 2
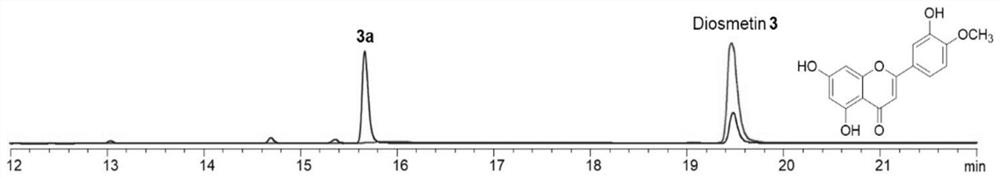
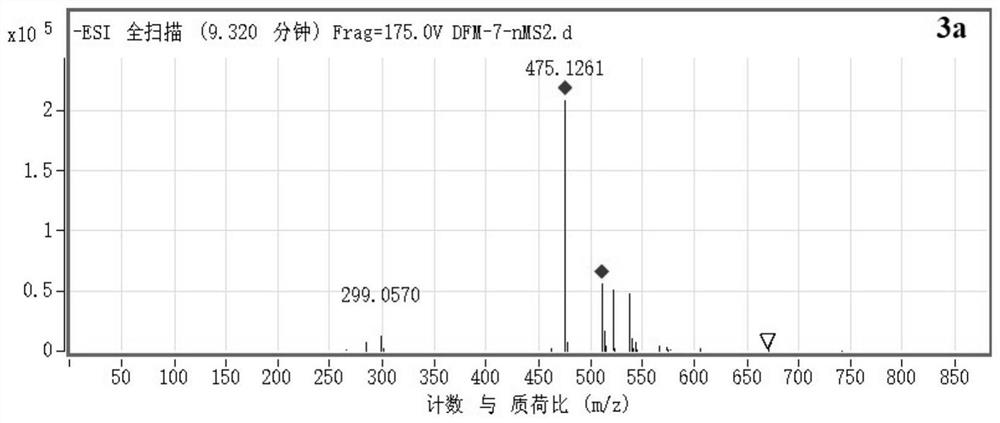
[0027] Example 2 Extraction, separation and structural identification of compound 3a
[0028] 2.1 Instruments and materials
[0029] Chromatographic pure water, chromatographic acetonitrile, analytical pure ethyl acetate, chromatographic or ordinary pure methanol, dichloromethane, etc. used in the laboratory were purchased from Fisher (USA); column chromatography silica gel (100-200 mesh) was purchased from Qingdao Ocean Chemical Plant Company. BUCHIDL-400 rotary evaporator, produced by Swiss BUCHI company. The circulating cooler is produced by Zhengzhou Great Wall Science, Industry and Trade Co., Ltd. The semi-automatic preparative liquid chromatograph is Agilent 1260Infinity II, and the C18 chromatographic column is Eclipse XDB-C18, 5μm, 9.4×250mm. The Agilent 1260 Infinity II analytical HPLC instrument equipped with a Poroshell 120SB-C18 reverse-phase column (2.7 μm, 4.6 mm×150 mm) was used for sample purity testing. No impurity peaks were required, and pure samples were...
Embodiment 3
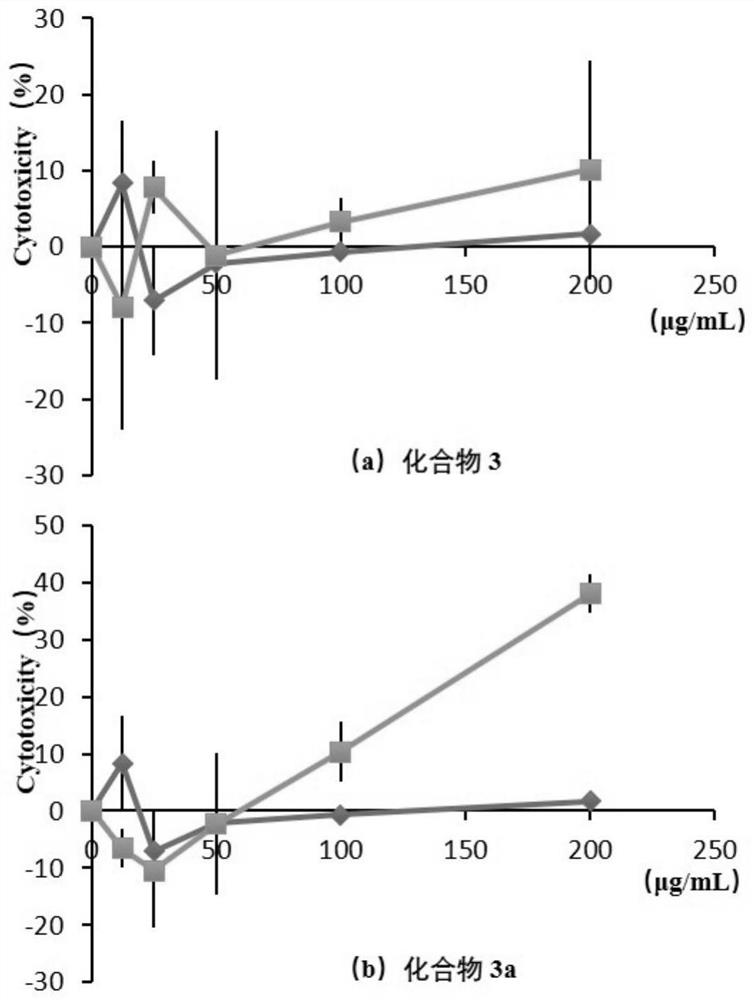
[0042] Example 3 Solubility test of diosmin and its glycosides
[0043] 3.1 Purpose of the experiment
[0044] Determination of the effect of sugar methylation on the solubility of naringenin in different solvents.
[0045] 3.2 Experimental method
[0046] Weigh 0.5mg of naringenin and compound 3 and 3a respectively, place in 500uL of different solvents at 25°C±2°C, shake vigorously for 30s every 5min; observe the dissolution within 30min, if no solute particles or liquid When dripping, it is considered to be completely dissolved. The selected solvents are distilled water, methanol, ethanol, etc.
[0047] 3.3 Experimental results and analysis
[0048] Table 2 shows the solubility of diosmin and its sugar methylated derivatives (compound 3a) in different solvents.
[0049] Table 2 Solubility of diosmin and compound 3a in different solvents
[0050]
[0051] Note: insoluble-; slightly soluble-+; partially soluble+; mostly soluble++; completely soluble+++
[0052] As sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com