Pharmaceutical composition containing poorly-soluble basic medicine
A composition and basic substance technology, applied in the direction of drug combination, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of dissolution, oral absorption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
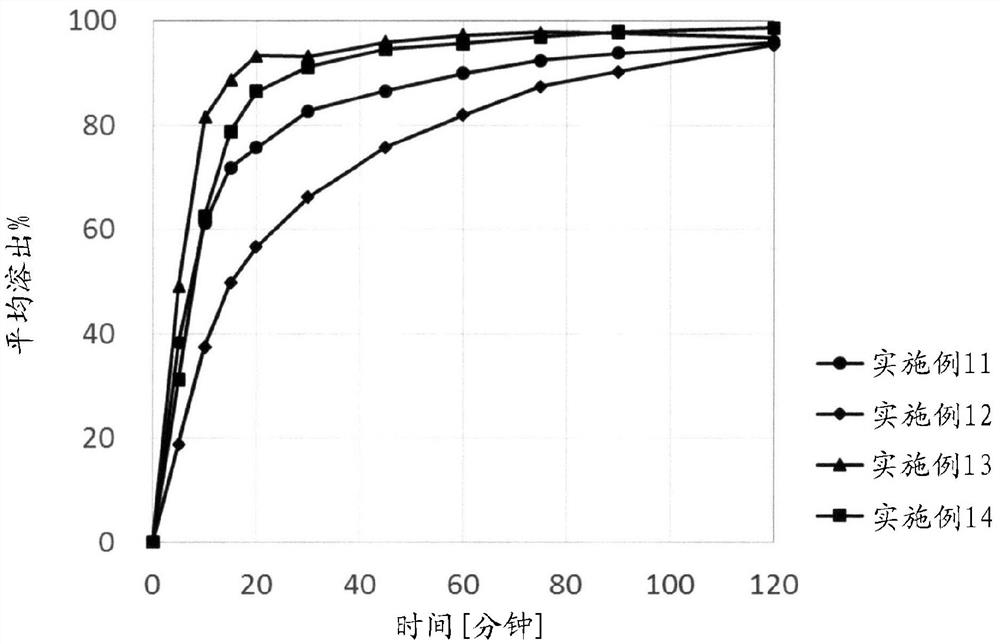
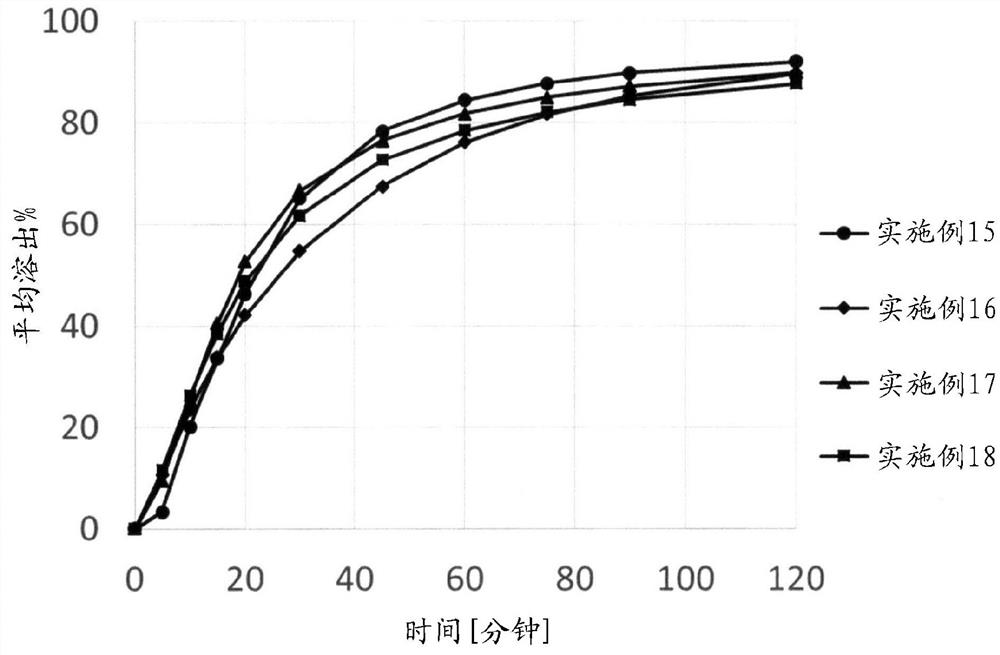
[0213] Examples are given below, and the present invention will be described in more detail, but the present invention is not limited to these Examples. In Examples 1-20, NIKKOL SLS (Nikko Chemicals Co., Ltd.) was used as sodium lauryl sulfate.
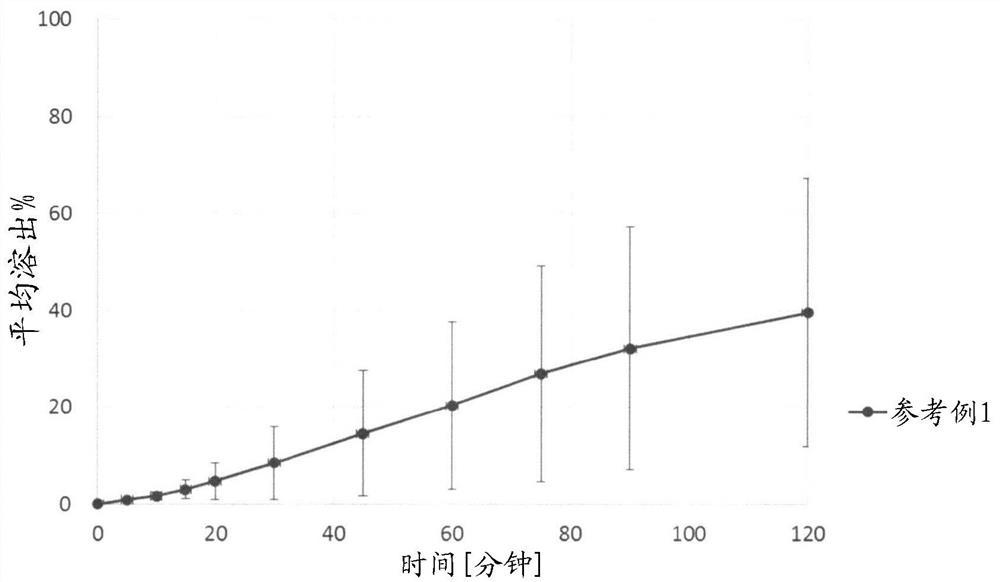
reference example 1
[0214] Reference Example 1: Formation of an impermeable coating
[0215] (manufacturing of preparations)
[0216] Tablets were prepared according to the amount of each component described in Table 1. The hydrochloride salt of the compound of formula (I) (hereinafter referred to as compound A), sodium lauryl sulfate, and magnesium stearate were mixed manually using a polyethylene container, respectively. The blended powder was compressed with a static compression tablet press (P-16, Riken Seiki Co., Ltd.) at a compression pressure (500 kgf) to produce a tablet (φ=9.0 mm) containing 150 mg of Compound A in terms of free form.
[0217] [Table 1]
[0218]
[0219] (Formulation evaluation and results)
[0220] For reference example 1, the test liquid uses 900mL of the Japanese Pharmacopoeia dissolution test liquid first solution comprising polyoxyethylene (10) octylphenyl ether 4%, and utilizes the Japanese Pharmacopoeia dissolution test paddle method to carry out the dissolu...
Embodiment 1-7
[0222] Examples 1-7: Research on disintegration of alkaline substances
[0223] (manufacturing of preparations)
[0224] Tablets were prepared according to the amount of each component of the tablet formulation described in Table 2. Each substance shown in Table 3 was used for X contained in the final powder component in the prescription. Mix the ingredients of the granule formulation, put the premixed powder into a stainless steel beaker, add purified water while stirring with a metal spatula, perform wet granulation, and use a vacuum dryer (VOS-301SD, Tokyo Rikaki Co., Ltd. ) to dry at room temperature. Next, it was sized by a sieve having a particle size diameter of 850 μm to form granules, and further mixed with the post-powder component to obtain a compounded powder. Tablets (15.9 x 8.4 mm) containing 300 mg of Compound A in terms of free form were produced by compressing each blended powder with a static compression tablet press (P-16, Riken Seiki Co., Ltd.) at a comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com