Synthesis method of cabozantinib and intermediate thereof
A technology of cabozantinib and its synthetic method, which is applied in the field of synthesis of cabozantinib and its intermediates, and can solve the problems of low production cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
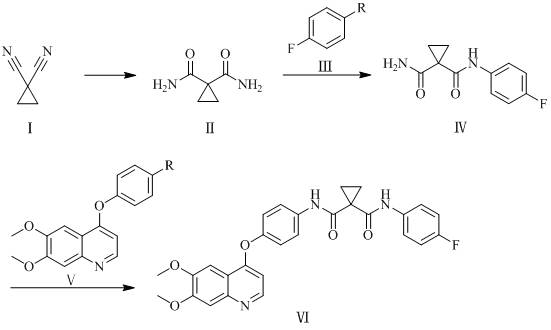
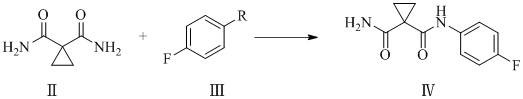
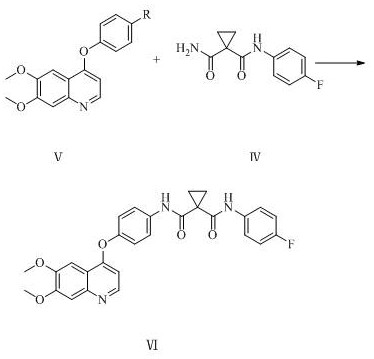
[0081] 1,1-Dicyanocyclopropane (9.2g, 0.1mol) was dissolved in 156ml of dichloromethane, tetrabutylammonium bisulfate (6.8g, 0.02mol) and potassium hydroxide aqueous solution (which contained potassium hydroxide 14.0g, 0.25mol), the temperature was controlled at 55°C to 60°C, and the reaction was carried out for 12h. After the reaction, the organic layer was washed with water, and the organic layer was taken, dried over sodium sulfate (5.0g) and spin-dried to obtain a white solid, which was washed with ethyl acetate ( 40ml) to obtain 1,1-dicarboxamidocyclopropane with a yield of 96.9% and a purity of 99.65%.
Embodiment 2
[0083] Dissolve 1,1-dicyanocyclopropane (9.2g, 0.1mol) in 184ml of dichloromethane, add 18 crown 6 (7.9g, 0.03mol) and potassium carbonate aqueous solution (which contains potassium carbonate 41.4g, 0.30mol ), the temperature was controlled at 60°C to 70°C, and the reaction was carried out for 10h. After the reaction, the organic layer was washed with water, the organic layer was taken, dried with sodium sulfate (5.0g) and spin-dried to obtain a white solid, which was beaten with ethyl acetate (40ml) to obtain 1 , 1-Diformamidocyclopropane, yield 95.3%, purity 99.54%.
Embodiment 3
[0085] Dissolve 1,1-dicyanocyclopropane (9.2g, 0.1mol) in 92ml of dichloromethane, add α-cyclodextrin (9.7g, 0.01mol) and lithium hydroxide aqueous solution (which contains lithium hydroxide 4.8 g, 0.20mol), the temperature is controlled at 15°C to 20°C, react for 20h, wash the organic layer with water after the reaction, take the organic layer, dry it with sodium sulfate (5.0g) and spin dry to obtain a white solid, wash with ethyl acetate (40ml ) beating to obtain 1,1-dicarboxamidocyclopropane with a yield of 94.6% and a purity of 99.12%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com