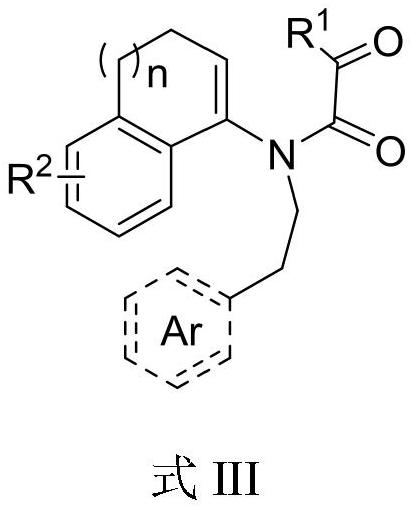
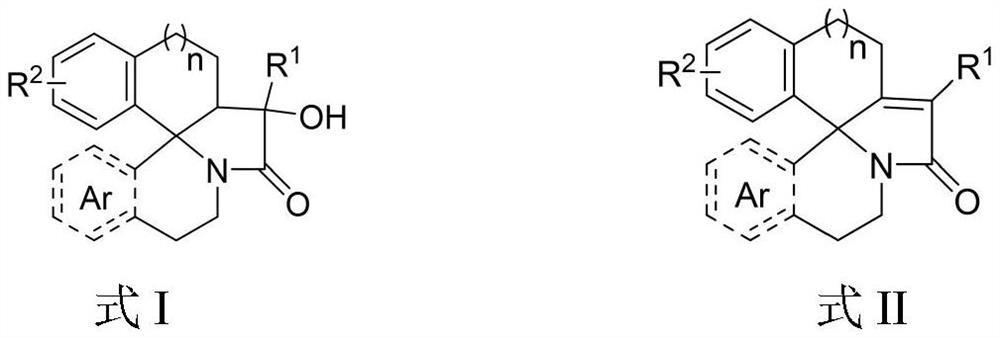
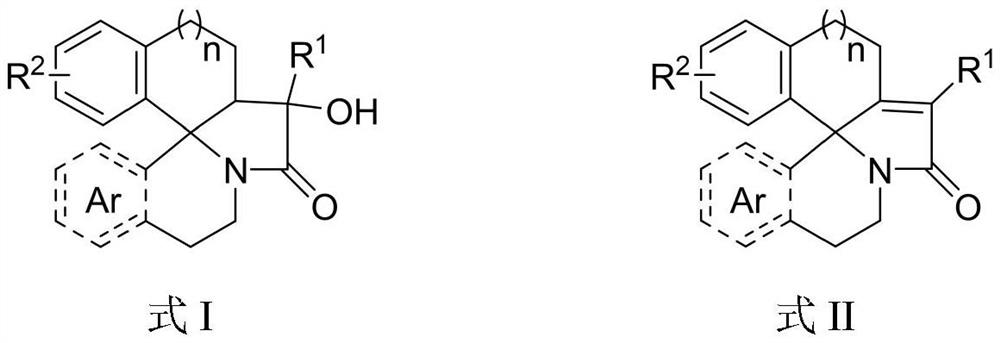Aza fused pentacyclic compound and preparation method thereof
A technology for cyclic compounds and compounds, applied in the field of aza-condensed pentacyclic compounds and their preparation, to achieve the effects of improving atom utilization efficiency, high yield, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, the compound shown in preparation formula IIIa structural formula
[0032] The reaction formula is as follows:
[0033]
[0034] Under the protection of argon, add tetralone (25mmol), 3,4-dimethoxyphenethylamine (25mmol), toluene 50mL ( MS molecular sieve drying treatment) and magnetons, after the system was refluxed for 24 hours, the toluene solvent was removed by rotary evaporation to obtain the imine without other treatment, which was directly used in the next step reaction.
[0035] The obtained imine (5 mmol) was dissolved in 10 mL of DMF (dried over molecular sieves) under the protection of argon, and triethylamine (6 mmol) was added. After cooling the reaction system to -40°C, the corresponding acetophenone chloride (5.5 mmol) was slowly added. Then keep it at -40°C for 30 minutes. After the reaction was completed, 20 mL of saturated aqueous sodium bicarbonate solution was added to the system to quench the reaction, extracted with ethyl aceta...
Embodiment 2
[0042] Embodiment 2, the compound shown in preparation formula IIIb structural formula
[0043] The reaction formula is as follows:
[0044]
[0045] According to the similar method described in Example 1, the compound represented by formula IIIb was prepared, and the two-step yield was 70%.
[0046] The compound is a white solid:
[0047] Melting point mp: 140-140°C;
[0048] 1 H NMR (500MHz, DMSO-d 6 )δ7.71-7.59(m,3H),7.48(t,J=5.0Hz,2H),7.09(d,J=8.2Hz,1H),6.92-6.91(m,2H),6.82-6.80(m ,2H),6.66(d,J=2.6Hz,1H),5.59(dd,J=6.4,2.9Hz,1H),4.57-4.51(m,1H),3.76(s,3H),3.75(s, 3H),3.73(s,3H),3.27-3.22(m,1H),2.94-2.82(m,2H),2.65-2.52(m,2H),2.10-2.04(m,1H),1.85-1.76( m,1H);
[0049] 13 C NMR (126MHz, DMSO-d 6 )δ190.9, 167.1, 158.5, 149.1, 148.0, 135.6, 135.1, 133.5, 131.3, 131.2, 131.0, 129.4, 129.4, 129.1, 129.0, 121.5, 113.5, 113.4, 115.552, 109.6, 57.3, 59.1 ,25.7,22.9;
[0050] HRMS (ESI) Calcd.for C 29 h 29 NO 5 Na,[M+Na] + 494.1938. Found: 494.1938.
[0051] It can b...
Embodiment 3
[0052] Embodiment 3, the compound shown in preparation formula IIIc structural formula
[0053] The reaction formula is as follows:
[0054]
[0055] According to the similar method described in Example 1, the compound represented by formula IIIc was prepared, and the two-step yield was 67%.
[0056] The compound is a white solid:
[0057] Melting point mp: 147-148°C;
[0058] 1 H NMR (400MHz, DMSO-d 6 )δ7.71-7.65(m,1H),7.64-7.60(m,2H),7.51-7.45(m,2H),7.41(dd,J=8.0,2.1Hz,1H),7.22(d,J= 2.1Hz, 1H), 7.14(dd, J=8.0, 0.9Hz, 1H), 6.95-6.88(m, 2H), 6.81(dd, J=8.1, 2.0Hz, 1H), 5.64(dd, J=6.3 ,3.0Hz,1H),4.52(dt,J=13.5,7.5Hz,1H),3.75(s,3H),3.72(s,3H),3.24(ddd,J=13.4,7.4,5.6Hz,1H) ,2.97-2.77(m,2H),2.68-2.53(m,2H),2.15-2.07(m,1H),1.88-1.78(m,1H);
[0059] 13 C NMR (126MHz, DMSO-d 6 )δ190.5, 166.5, 148.7, 147.5, 135.9, 134.7, 134.2, 132.9, 132.2, 131.9, 130.8, 130.4, 129.8, 129.0, 128.9, 125.3, 121.0, 119.4, 113.50, 114.8, 5.2, 52.5 ,21.9;
[0060] HRMS (ESI) Calcd.for C 28 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com