A nitrogen-doped ferrous sulfide redox catalyst material and its preparation and application
A catalyst, nitrogen doping technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of poor stability, scarcity of resources, expensive Pt-based cathode catalysts, etc., and achieve low prices. , high catalytic activity, abundant reserves effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example example 1
[0029] Catalyst preparation
[0030] a, 6.3924g of sodium diethyldithiocarbamate and 1.7973g of ferrous chloride are dissolved in 100mL deionized water, react rapidly to obtain iron diethyldithiocarbamate (II) black precursor precipitate; b 1. Stir the liquid in step a at room temperature for more than 1 hour to allow it to fully precipitate, then use deionized water and absolute ethanol to wash and filter it several times, and dry it in a blast drying oven at 60°C for 12 hours to obtain Black precursor powder;
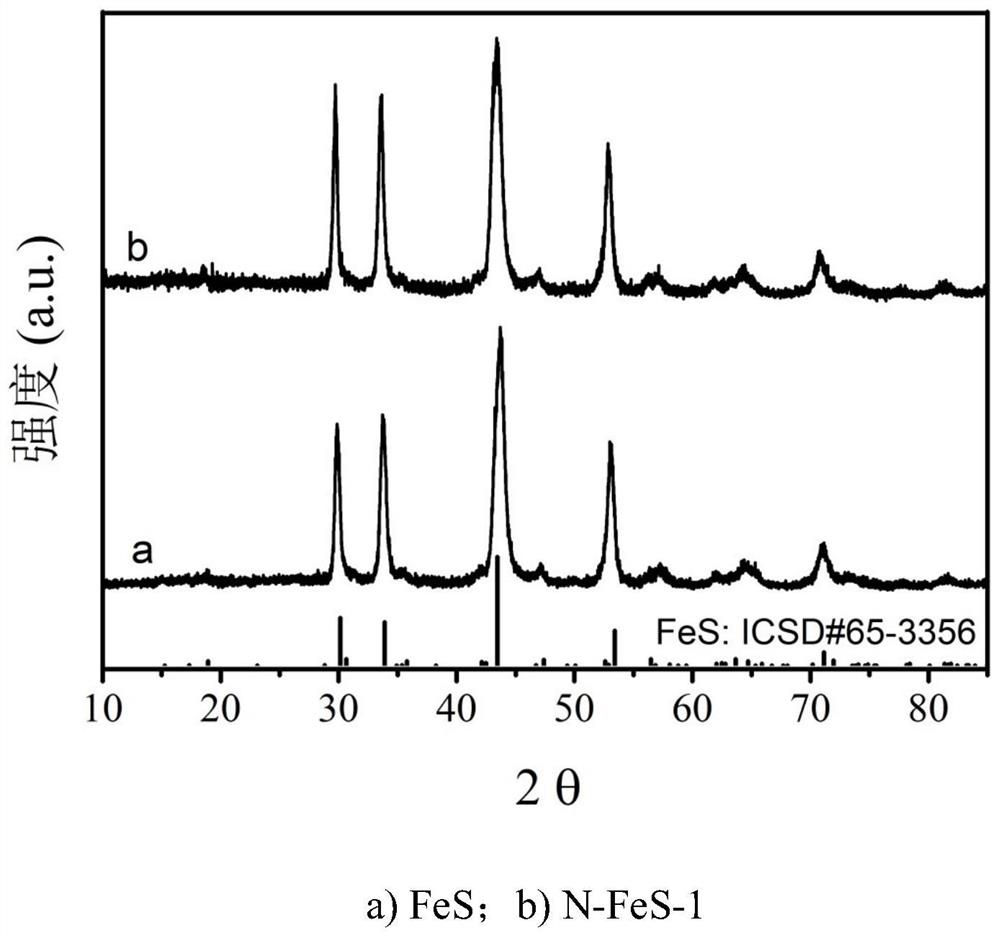
[0031] c. Put the precursor powder obtained in step b into a sealed tube furnace, and calcinate at 700° C. for 2 hours under an argon atmosphere, and the heating rate is 5° C. / min. Naturally cool down to room temperature to obtain a flower-shaped FeS microsphere catalyst;
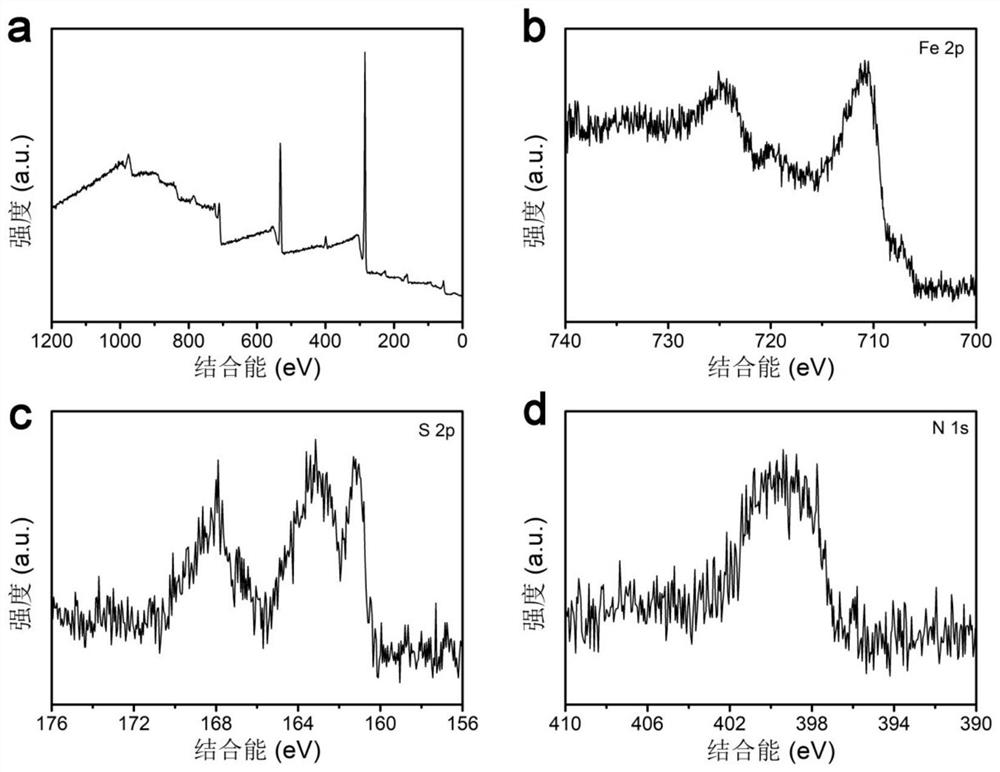
[0032] d. Place the FeS powder obtained in step c again in a sealed tube furnace, and calcinate at 400° C. for 1 h under an ammonia atmosphere, and the heating rate is 5° C. / min. After naturally...
example example 2
[0037] Catalyst preparation
[0038] a, 6.3924g of sodium diethyldithiocarbamate and 1.7973g of ferrous chloride are dissolved in 100mL deionized water, react rapidly to obtain iron diethyldithiocarbamate (II) black precursor precipitate; b 1. Stir the liquid in step a at room temperature for more than 1 hour to allow it to fully precipitate, then use deionized water and absolute ethanol to wash and filter it several times, and dry it in a blast drying oven at 60°C for 12 hours to obtain Black precursor powder;
[0039] c. Put the precursor powder obtained in step b into a sealed tube furnace, and calcinate at 700° C. for 2 hours under an argon atmosphere, and the heating rate is 5° C. / min. Naturally cool down to room temperature to obtain a flower-shaped FeS microsphere catalyst;
[0040] d. Place the FeS powder obtained in step c again in a sealed tube furnace, and calcinate at 500° C. for 1 h under an ammonia atmosphere, and the heating rate is 5° C. / min. After naturally...
example example 3
[0044] Catalyst preparation
[0045] e, 6.3924g of sodium diethyldithiocarbamate and 1.7973g of ferrous chloride were dissolved in 100mL of deionized water, reacted quickly to obtain a black precursor precipitate of diethyldithiocarbamate iron (II);
[0046] f. Stir the liquid in step a at room temperature for more than 1 hour to allow it to fully precipitate, then use deionized water and absolute ethanol to wash and filter it several times, and dry it in a blast drying oven at 60°C for 12 hours. Obtain black precursor powder;
[0047] g. Put the precursor powder obtained in step b into a sealed tube furnace, and calcinate at 700° C. for 2 hours under an argon atmosphere, and the heating rate is 5° C. / min. Naturally cool down to room temperature to obtain a flower-shaped FeS microsphere catalyst;
[0048] Electrochemical performance test
[0049] The test method is the same as in Example 1, and the specific rotating disk electrode polarization curve diagram is shown in Fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com