Medical quantitative inhalation aerosol
An aerosol and preparation technology, applied in the field of metered dose inhalation aerosols, can solve the problem of not being able to solve the problem of degradation well, and achieve the maintenance and enhancement of physical stability, significant physical stability, and enhanced chemical and physical stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In this experiment, the influence of different combinations of pressure-resistant containers and different sealing gasket metering valves on the accelerated chemical stability of ipratropium bromide medical quantitative inhalation aerosol was investigated.
[0037] Combination type of container tank-metering valve: non-coated aluminum tank-EPDM sealing gasket metering valve, fluorocarbon polymer coated aluminum tank-EPDM sealing gasket metering valve, stainless steel tank-EPDM sealing gasket metering valve Rubber Gasket Metering Valve; Uncoated Aluminum Can-Chlorobutyl Rubber Gasket Metering Valve, Fluorocarbon Polymer Coated Aluminum Can-Chlorobutyl Rubber Gasket Metering Valve, Stainless Steel Tank-Chlorobutyl Rubber Seal Gasket metering valve.
[0038] Aerosol preparations: In order to accurately investigate the impact of the container-metering valve combination on the stability, a single variable is controlled in this embodiment 1. All the above-mentioned container-...
Embodiment 2
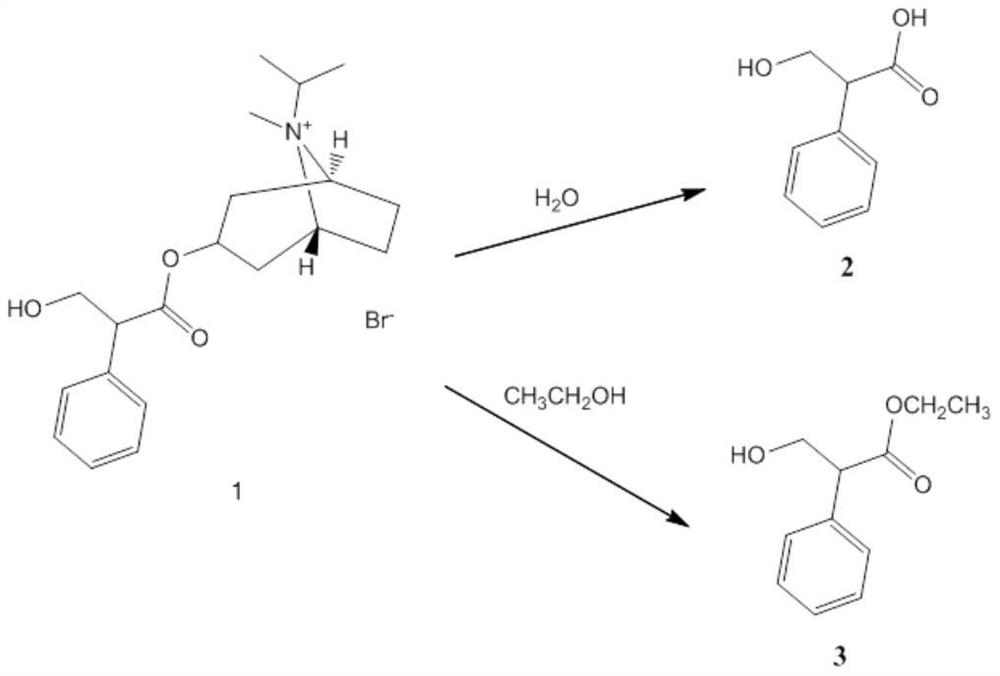
[0050] This experiment compares the ipratropium bromide solution-type aerosol combined with the metering valve combination of non-coated aluminum can and chlorobutyl rubber sealing gasket with the commercially available ipratropium bromide solution-type aerosol of Boehringer Ingelheim Pharmaceutical Company The chemical and physical stability of the agent in the accelerated test.
[0051] According to reference 4 (Boehringer Ingelheim, "Ipratropium bromide aerosol manual"), With stainless steel tank - metering valve combination with gasket of unknown material.
[0052] Comparison group: commercially available ipratropium bromide solution aerosol from Boehringer Ingelheim Pharmaceutical Company It adopts the metering valve combination of stainless steel tank and unknown gasket, and sets upright comparison group and inverted comparison group respectively.
[0053] Experimental group: ipratropium bromide solution-type aerosol of the present invention, it adopts non-coated...
Embodiment 3
[0068] The influence of different tank-metering valve combinations on the physical stability of ipratropium bromide solution aerosol is also reflected in the measurement of aerodynamic particle size distribution (Aerodynamic Particle Size Distribution, APSD).
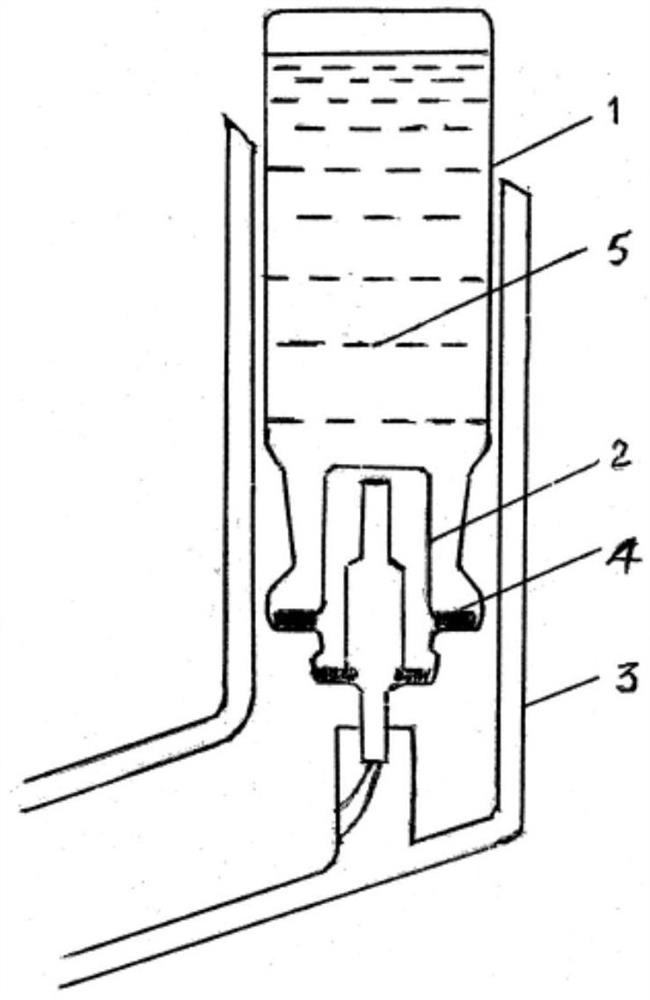
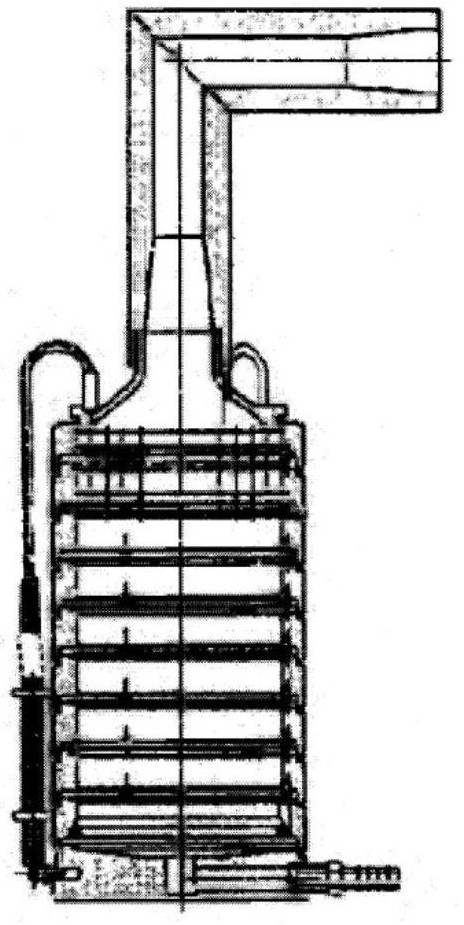
[0069]According to the guidelines of reference 6 ("Guiding Principles for Quality Control Opinions on Metered-Dose Inhaler and Dry Powder Inhaler Products" issued by the US FDA in April 2018), the aerodynamic particle size distribution is determined using "Quality by Design (QbD) )" concept to develop a "critical quality attribute" (Critical Quality Attribution, CQA) in metered dose inhalation aerosol products. The Anderson Cascade Impactor (ACI, figure 2 Its structure is shown, including 8 grades - 0-7 grades and the last layer of filter paper "F layer") to determine the aerodynamic particle size distribution (Reference 7: Chinese Pharmacopoeia 2015 edition, "Inhalation preparations fine particle air Kinetic Properti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com