Preparation method of paliperidone palmitate suspension
A technology of paliperidone and palmitic acid, which is applied in the field of preparation of paliperidone palmitate suspension, can solve the problems of high risk of organic solvent residue, long production time, medium residue, etc., and avoid the problem of solvent residue , Improve safety, mild condition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0045] A preparation method of paliperidone palmitate suspension, comprising the following steps:
[0046] (1) Dispersing and dissolving the paliperidone palmitate drug particles in a first water-soluble good solvent containing a surfactant to obtain a drug solution; the first water-soluble good solvent is an acid solution. The acid solution is an acetic acid solution, and the concentration of the acetic acid solution is 30-80%. The weight percent content of the surfactant in the first water-soluble good solvent containing the surfactant is 1.2-3.6%.
[0047] (2) the drug solution is slowly added to the second water-soluble solvent containing the stabilizer, the drug solution is added to the second water-soluble solvent containing the stabilizer while adding energy to make the drug particles precipitate rapidly, and the resuspended can be mixed Suspension; remove acetic acid from the suspension through one or more of heating evaporation, vacuum removal and rotary evaporation,...
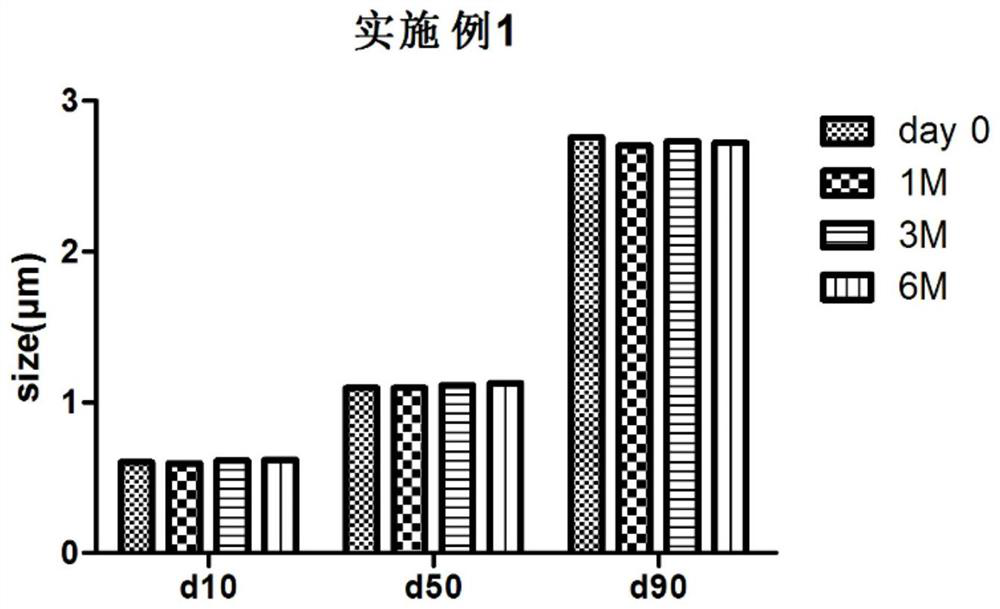
Embodiment 1
[0049] Preparation of a suspension with a final concentration of the active substance (pharmaceutical ingredient) of approximately 1%:
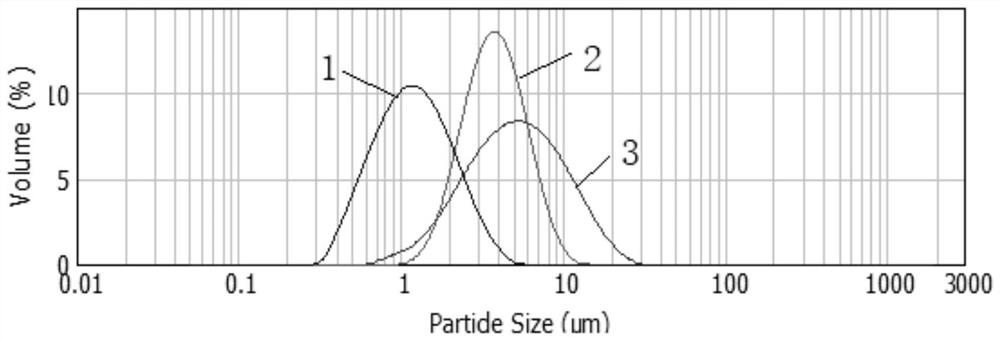
[0050] 5g of glacial acetic acid and 0.6g of polysorbate 20 were stirred and dissolved in 6g of ultrapure water in turn. After the solution was clear, 5g of paliperidone palmitate particles (average particle size of about 5 μm) were dispersed and dissolved in the above solution at room temperature. In the aqueous solution, stir and mix well until the solution is clear;
[0051] The above dissolved clear solution was added dropwise through a peristaltic pump (Cole-Parmer, 07523-80) and an injection needle (the speed was controlled so that the solubility of the dissolved hydrophobic drug particles was rapidly reduced and precipitated) to 450 g containing 2% polysorbate. 20 °C in ice water (the water temperature is controlled at 0-3 °C), and at the same time, high shear (IKA, T25 shear head) is added to the ice water bath, and the rotation speed...
Embodiment 2
[0054] Preparation of a suspension with a final concentration of active substances of approximately 5%:
[0055] 5g of glacial acetic acid and 0.45g of polysorbate 20 were stirred and dissolved in 2g of ultrapure water in turn. After the solution was clear, 5g of paliperidone palmitate particles (average particle size of about 76 μm) were dispersed and dissolved in the above solution at room temperature. In the aqueous solution, stir and mix well until the solution is clear;
[0056] The above dissolved clear solution was added dropwise through a peristaltic pump (Cole-Parmer, 07523-80) and an injection needle (the speed was controlled so that the solubility of the dissolved hydrophobic drug particles was rapidly reduced and precipitated) to 85 g containing 3% polyethylene glycol. Alcohol 4000 in ice water (the water temperature is controlled at 0-3 ℃), and high shear (IKA, T25 shear head) is added to the ice water bath at a speed of 13000 rpm; during the dropping process, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com