Nano-micelles activated by short-time near-infrared illumination for rapidly releasing medicines
A near-infrared light and nanomicelle technology is applied in the preparation of nanomicelles and the application field of rapid drug release, which can solve the problems of slow drug release rate, thermal damage to cells and tissues, unfavorable clinical application, etc., and achieves short illumination time. , good stability and high drug release efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
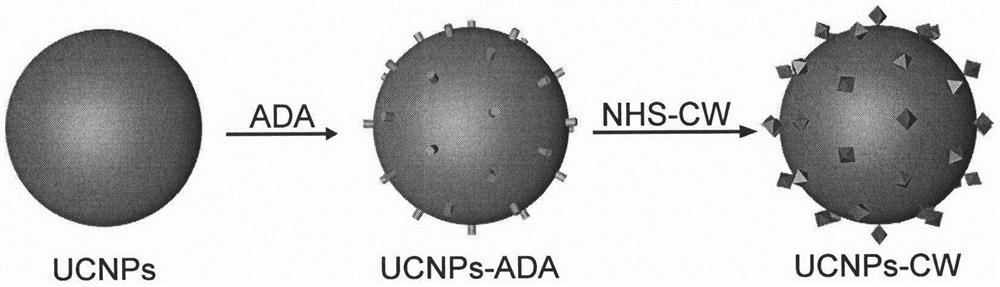
[0020] Example 1: Combining image 3 , Synthesis of 800CW modified upconversion nanoparticles UCNPs-CW.
[0021] 200mg NaYF 4 :Gd@NaYF 4 : Er, Yb@NaYF 4 : Yb, Nd(UCNPs) dispersed in 10mL CHCl 3 Add 50mg of alendronic acid (ADA), 6mL of pure water and 4mL of ethanol, and adjust the pH value of the mixture to about 2 with 1M hydrochloric acid. After stirring at room temperature for 30 minutes, the upper aqueous phase was collected, centrifuged to obtain UCNPs-ADA, washed several times with pure water, and dispersed in 10 mL of pure water. Then mix 5mL 1mg / mL UCNPs-ADA with 3μL 0.5mM Mixed and stirred overnight at room temperature, centrifuged and washed several times with THF, dispersed in 5 mL THF (Solution A). After mixing 2 μL octanoic acid with 100 μL EDC (0.1M), 100 μL NHS (0.1M) and 300 μL THF and stirring at room temperature for 2 hours, it was added to 5 mL of solution A and stirred at room temperature for 8 h. The obtained hydrophobic UCNPs-CW were centrifuged, ...
Embodiment 2
[0022] Example 2: Combining Figure 4 , Synthesis of amphiphilic polymer long-chain P-DASA.
[0023] Add 15.48mmol n-dodecylamine and 15.48mmol n-dodecyl isocyanate to 130mL CH 2 Cl 2 , stirred at room temperature for 1 h under nitrogen protection, then added 15.48 mmol of malonyl chloride and heated to reflux for 1 h. After cooling to room temperature, add 60mL of 1M hydrochloric acid and mix well, and the crude product is washed with CH 2 Cl 2 extraction. The organic phase was washed with water and MgSO 4 Drying and purification by column chromatography (hexane: ethyl acetate = 9:1 to 4:1) gave product 1.
[0024] 13.6 mmol of 2-furan aldehyde was added to 27 mL of an aqueous solution containing 13.6 mmol of compound 1, and stirred at room temperature for 10 h. use CH 2 Cl 2 Extraction product (compound 2), organic phase is passed through MgSO 4 Dry, filter and dry in vacuo.
[0025] 2.32 mmol of 6-azido-N-ethylhexan-1-amine was added to 5 mL of a THF solution con...
Embodiment 3
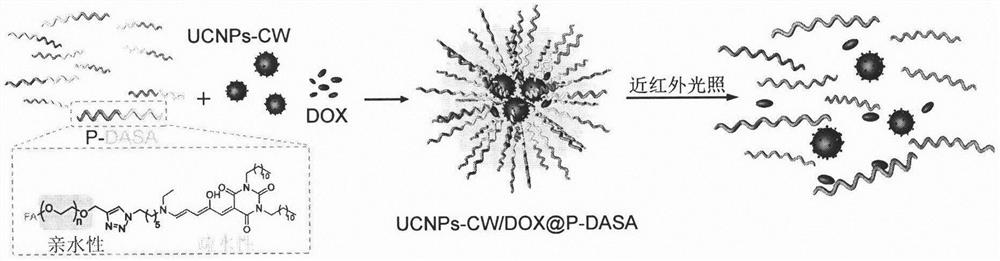
[0027] Example 3: Binding figure 1 , Synthesis of upconversion nanomicelle UCNPs-CW / DOX@P-DASA.
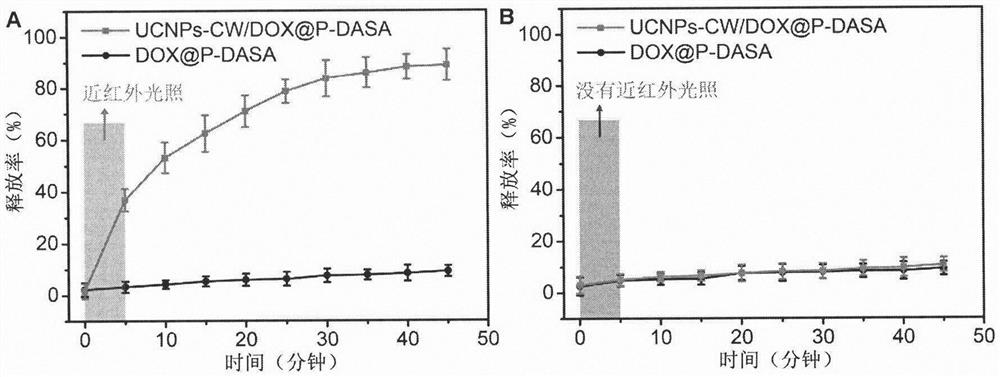
[0028] 2 mg DASA-PEG was dispersed in 2.7 mL THF, mixed with 1 mg UCNPs-CW and 100 μL 0.4 mg / mL DOX and sonicated for 20 min. The mixture was then added dropwise to 60 mL of water and stirred at 37 °C for 8 h. The generated upconversion nanomicelle UCNPs-CW / DOX@P-DASA was collected by centrifugation (4000r, 5 minutes) and dispersed in 2 mL of water.
[0029] Nanomicelle DOX@P-DASA without UCNPs-CW was synthesized using a similar method, and UCNPs-CW was not added during the synthesis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com