Gamma-hydrazino cyanide compound and synthesis method thereof
A technology for a cyanide compound and a synthesis method, which is applied in the field of γ-hydrazine cyanide compounds and the synthesis thereof, can solve problems such as blank synthesis methods, and achieve the effects of simple operation, mild reaction conditions and strong compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] A kind of synthetic method of described gamma-hydrazine cyanide compound comprises following synthetic steps:
[0056] (1) Under inert gas protection conditions, azodicarboxylate and α-iminooxy acid are mixed and dissolved in a solvent (acetonitrile, tetrahydrofuran, toluene, acetone, 1,4-dioxane, dichloromethane, 1,2-dichloroethane or N,N-dimethylformamide), under the action of cerium chloride heptahydrate and alkali (cesium carbonate, potassium carbonate or sodium carbonate), react for 36-48h to obtain the formula The γ-hydrazine cyanide of structure shown in I; The ratio of the amount of substance of described α-iminooxyacid, azodicarboxylate, catalyst, alkali is 1: 1-3: 0.05-0.15: 0.2- 1;
[0057] The α-iminooxyacid has a structure shown in formula II:
[0058]
[0059]
[0060] Wherein, the definitions of R1, R2, Y and n are consistent with those in the formula I.
[0061] The azodicarboxylate has the structure shown in formula III~1-III~4:
[0062]
...
Embodiment 1
[0066]
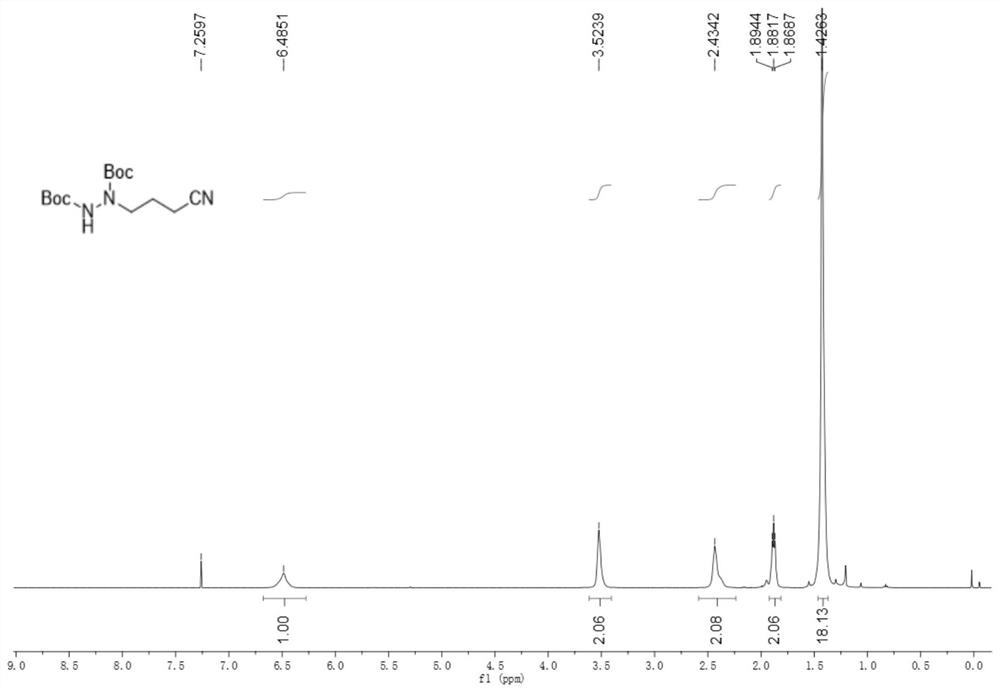
[0067] 2-((cyclobutenylamino)oxy)-2-methylpropionic acid (34.2mg, 0.2mmol), di-tert-butyl azodicarboxylate (69.1mg, 0.3mmol), cerium chloride heptahydrate (7.4mg, 0.02mmol) and potassium carbonate (5.5mg, 0.04mmol) were added to an argon-protected reaction flask, and finally acetonitrile (2.0mL) was added, and then reacted at room temperature under 460nm blue light for 48h. Chromatography (eluent: petroleum ether / ethyl acetate volume ratio 5:1) isolated 42.1 mg with a yield of 70%.
[0068] like figure 1 As shown, product characterization: white solid; m.p.59-60°C; 1H NMR (500MHz, CDCl3) δ6.49(m, 1H), 3.52(m, 2H), 2.43(m, 2H), 1.88(t, J =6.35Hz, 2H), 1.43(s, 18H)
Embodiment 2
[0070]
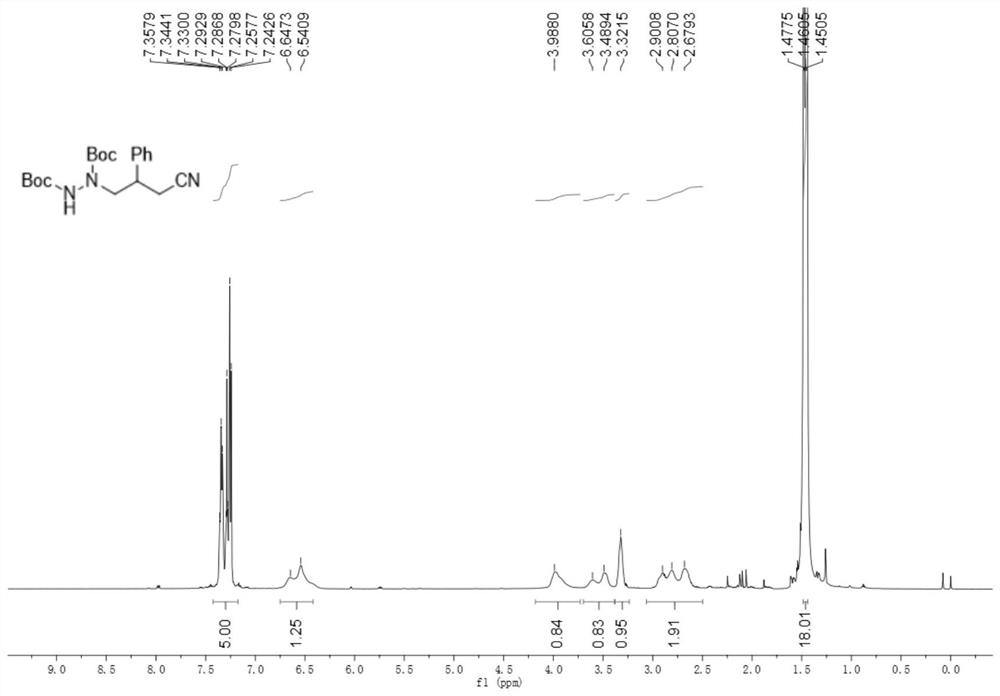
[0071] 2-Methyl-2-(((3-phenylcyclobutenyl)amino)oxy)propanoic acid (49.4mg, 0.2mmol), di-tert-butyl azodicarboxylate (69.1mg, 0.3mmol) , cerium chloride heptahydrate (7.4mg, 0.02mmol) and potassium carbonate (5.5mg, 0.04mmol) were added to the reaction flask protected by argon, and finally acetonitrile (2.0mL) was added, and then reacted at room temperature under 460nm blue light for 48h , after the reaction, separated by column chromatography (eluent: petroleum ether / ethyl acetate volume ratio of 5:1) to obtain 51.4mg, the yield was 68%.
[0072] like figure 2 As shown, product characterization: brown oil; 1H NMR (500MHz, CDCl3) δ7.36-7.24 (m, 5H), 6.65-6.54 (m, 1H), 3.99 (m, 1H), 3.61-3.49 (m, 1H ), 3.32 (s, 1H), 2.90-2.68 (m, 2H), 1.46 (t, J=8.5Hz, 18H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com