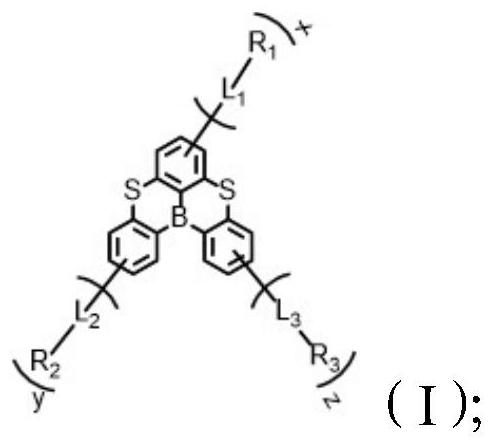
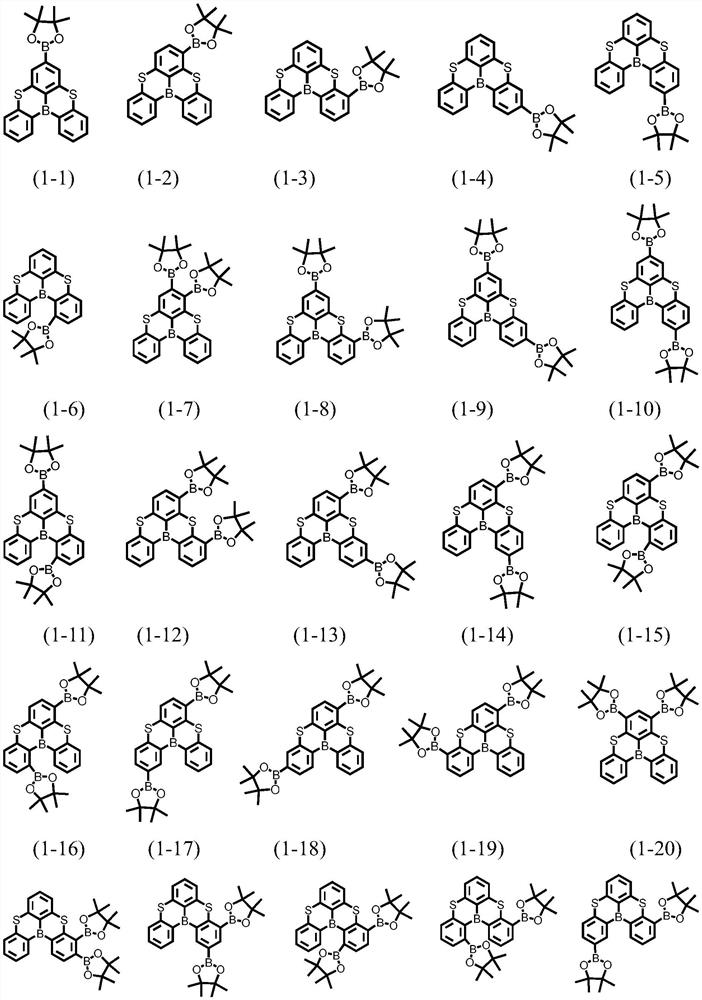
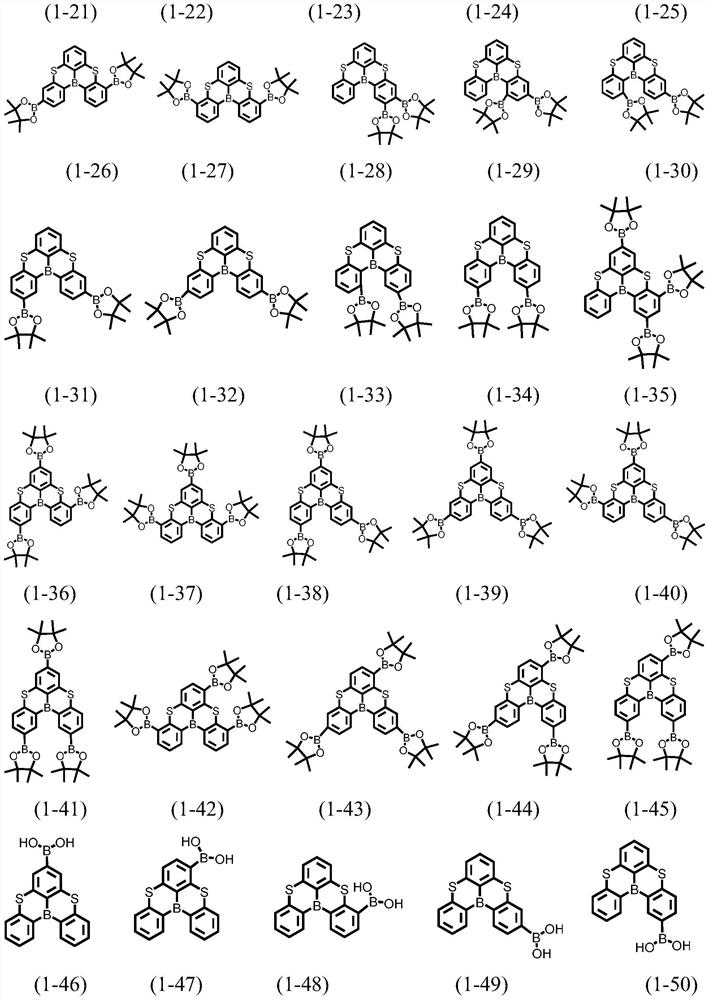
A kind of condensed ring compound containing boron atom and sulfur atom and its preparation method and application
A compound and fused ring technology, applied in the field of fused ring compounds and their preparation, can solve the problems of reduced external quantum efficiency of devices and complex device structures, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071]
[0072] Under an argon atmosphere, add m-1 (30.0g, 0.11mol) and sodium thiophenate (43.6g, 0.33mol) to a 500mL three-necked flask, add 180mL of N-methylpyrrolidone (NMP) to the bottle, and heat up to 150°C, stirred and reacted for 10 hours under the protection of argon, then cooled to room temperature, diluted the reaction solution with toluene and poured it into water, separated the organic phase, added anhydrous sodium sulfate to dry, filtered the obtained organic phase to remove the solvent, The crude product was separated by column to obtain the product m-2 (23.0 g, yield: 46%).
[0073] Elemental analysis structure (C 18 h 12 Br 2 S 2 ): theoretical value C, 47.81; H, 2.67; S, 14.18 test value C, 47.72; H, 2.73; S, 14.26.
[0074] Electrospray mass spectrometry (ESI-MS): theoretical value 452.2; experimental value 452.2 (M + ).
[0075] Under argon atmosphere, m-2 (5.0g, 11.1mmol) and dry o-xylene (80mL) were added to a 250mL two-necked flask, and butylli...
Embodiment 2
[0082]
[0083]Under an argon atmosphere, add m-4 (10.0g, 0.05mol) and sodium thiophenate (16.5g, 0.13mol) to a 250mL three-necked flask, add 80mL of N-methylpyrrolidone (NMP) to the bottle, and heat up to 150°C, stirred and reacted for 10 hours under the protection of argon, then cooled to room temperature, diluted the reaction solution with toluene and poured it into water, separated the organic phase, added anhydrous sodium sulfate to dry, filtered the obtained organic phase to remove the solvent, The crude product was separated by column to obtain the product m-5 (4.5 g, yield: 24%).
[0084] Elemental analysis structure (C 18 h 13 BrS 2 ): theoretical value C, 57.91; H, 3.51; S, 17.18 test value C, 57.96; H, 3.53; S, 17.18.
[0085] ESI-MS: theoretical value 372.0; experimental value 372.1 (M + ).
[0086] Under argon atmosphere, m-5 (1.5g, 4.0mmol) and dry o-xylene (70mL) were added to a 250mL two-necked flask, and butyllithium solution (1.7mL, 2.5 M, 4.2mmol), ...
Embodiment 3
[0097]
[0098] Under an argon atmosphere, magnesium chips (0.14g, 5.78mmol) were added to a 100mL two-necked flask, and m-3 (2.0g, 5.25mmol) was dissolved in 30mL of dry THF solution and added dropwise to a flask containing magnesium In a two-necked flask with crumbs, filter the obtained Grignard reagent, and slowly add it dropwise to a THF solution of trimethyl borate (0.82g, 7.9mmol) at -70°C. After the reaction, slowly pour the reaction solution into the fast In a stirring ice-water bath, add hydrochloric acid and stir for 30 min, the mixture is extracted with ether, the organic phase is washed with deionized water, dried with anhydrous sodium sulfate, the organic phase is concentrated and dried, and the crude product is recrystallized with hot n-hexane The product 1-46 was obtained (1.3 g, yield: 71%).
[0099] Elemental analysis structure (C 18 h 12 B 2 o 2 S 2 ): theoretical value C, 62.48; H, 3.50; S, 18.53 test value C, 62.41; H, 3.53; S, 18.52.
[0100] ESI-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| full width at half maximum | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com