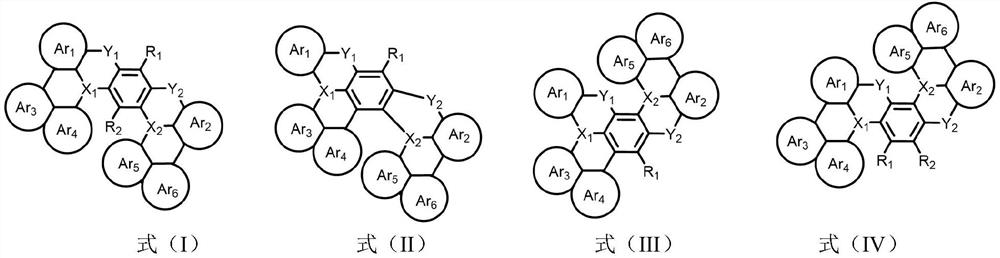
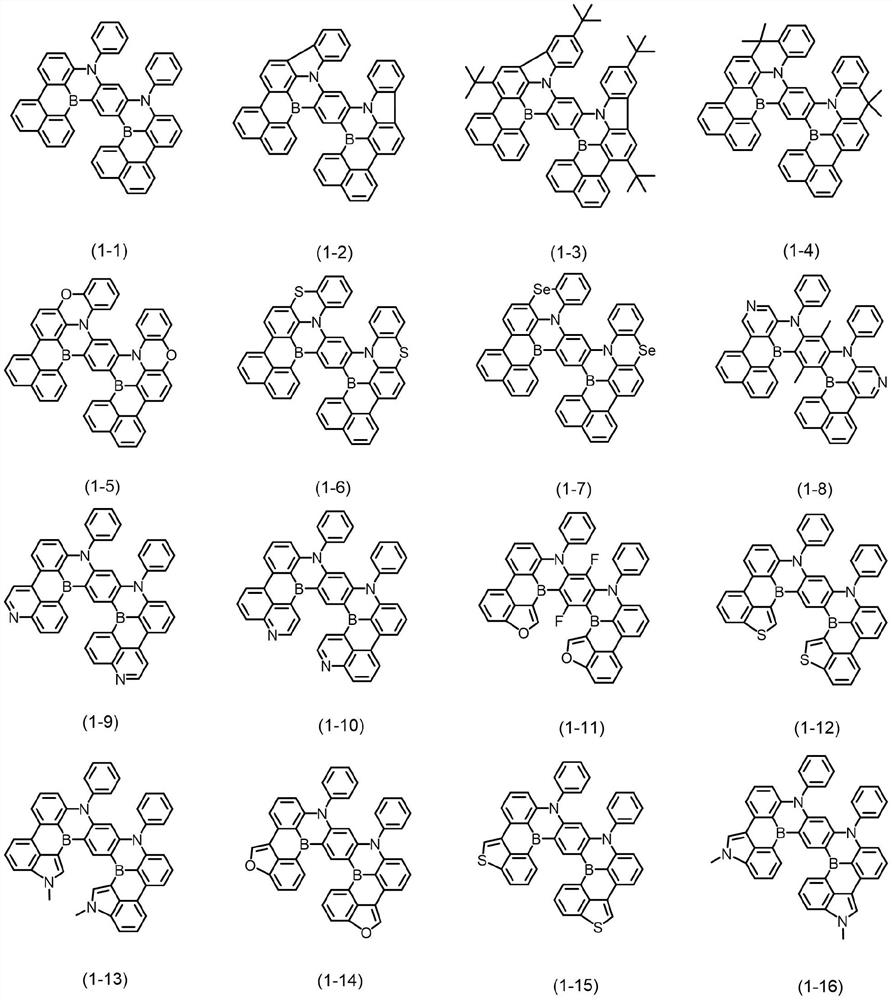
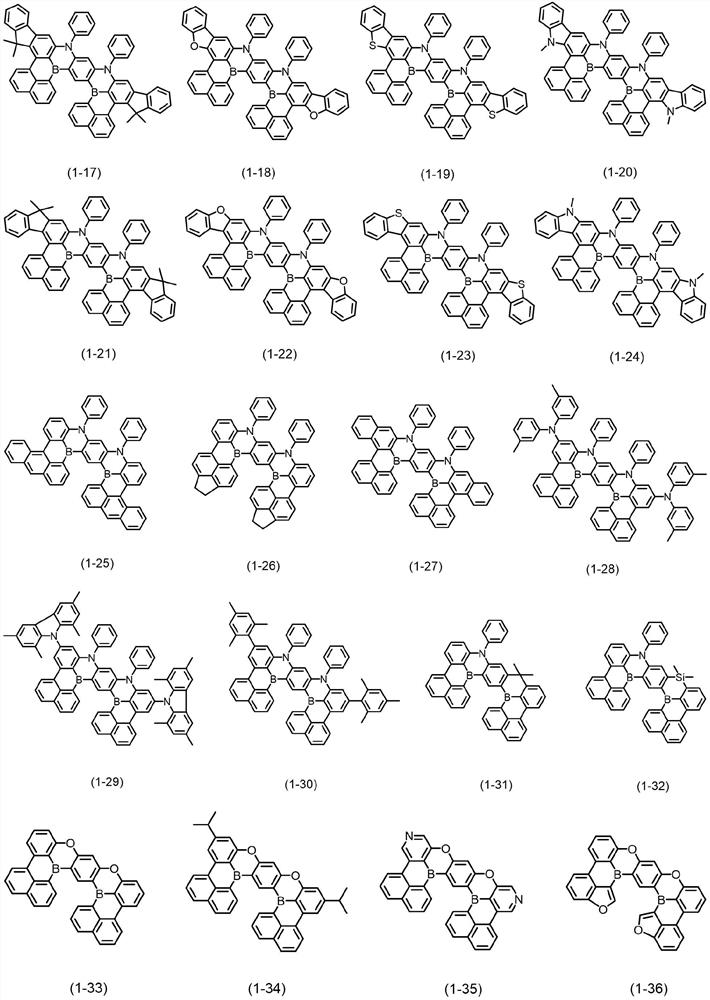
Bora or phosphorus heterocyclic fused ring compound, preparation method thereof and luminescent device
A compound and condensed ring technology, which is applied in the fields of boron hetero or phosphor hetero condensed ring compounds and their preparation methods and light-emitting devices, can solve the problems of complex device structure and reduced external quantum efficiency of the device, and achieve high light-emitting efficiency, mild conditions, simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059]
[0060] Under argon atmosphere, m-1 (3.2g, 10.0mmol), 1-boronic naphthalene (1.7g, 10.0mmol), potassium carbonate (1.9g, 20.0mmol) and Pd(PPh 3 ) 2 Cl 2 (0.18g, 0.25mmol), 30mL of toluene was added, and the system was reacted at 120°C for 5h. After cooling to room temperature, deionized water was added thereto, and the organic phase extracted was dried with anhydrous sodium sulfate, and the solvent was removed. The crude product was separated by silica gel column chromatography to obtain product m-2 (3.2 g, yield: 44%).
[0061] Elemental analysis structure (C 16 h 10 BrCl): Theoretical: C, 60.51; H, 3.17; Found: C, 60.54; H, 3.12.
[0062] MALDI-TOF-MS: theoretical 316.0; experimental 316.0.
[0063] Under argon atmosphere, m-2 (3.2g, 10.0mmol), aniline (0.9g, 10.0mmol), sodium tert-butoxide (1.9g, 20.0mmol) and (AMPHOS) were added to a 250mL two-necked flask 2 PdCl 2 (0.35g, 0.5mmol), add o-xylene 60mL, and react the system at 120°C for 5h. After cooling t...
Embodiment 2
[0073]
[0074] Under argon atmosphere, m-5 (4.4g, 10.0mmol), 1-boronic naphthalene (1.7g, 10.0mmol), potassium carbonate (1.9g, 20.0mmol) and Pd(PPh 3 ) 2 Cl 2 (0.18g, 0.25mmol), 30mL of toluene was added, and the system was reacted at 120°C for 5h. After cooling to room temperature, deionized water was added thereto, and the organic phase extracted was dried with anhydrous sodium sulfate, and the solvent was removed. The crude product was separated by silica gel column chromatography to obtain product m-6 (1.8 g, yield: 40%).
[0075] Elemental analysis structure (C 30 h 30 ClN): Theoretical: C, 81.89; H, 6.87; N, 3.18; Found: C, 81.86; H, 6.83; N, 3.14.
[0076] MALDI-TOF-MS: theoretical 439.2; experimental 439.2.
[0077] Under argon atmosphere, add m-6 (4.4g, 10.0mmol), m-difluorobenzene (0.6g, 5.0mmol), cesium carbonate (6.5g, 20.0mmol) into a 250mL two-necked flask, add DMF 60mL, The system was reacted at 120°C for 5h. After being cooled to room temperature, dic...
Embodiment 3
[0084]
[0085] Under argon atmosphere, add m-8 (3.2g, 10.0mmol), 1-boronic acid naphthalene (1.7g, 10.0mmol), potassium carbonate (1.9g, 20.0mmol) and Pd(PPh 3 ) 2 Cl 2 (0.18g, 0.25mmol), 30mL of toluene was added, and the system was reacted at 120°C for 5h. After cooling to room temperature, deionized water was added thereto, and the organic phase extracted was dried with anhydrous sodium sulfate, and the solvent was removed. The crude product was separated by silica gel column chromatography to obtain product m-9 (1.6 g, yield: 49%).
[0086] Elemental analysis structure (C 15 h 9 BrClN): Theoretical: C, 56.55; H, 2.85; N, 4.40; Found: C, 56.58; H, 2.82; N, 4.43.
[0087] MALDI-TOF-MS: theoretical 317.0; experimental 317.0.
[0088] Under argon atmosphere, m-9 (3.2g, 10.0mmol), aniline (0.9g, 10.0mmol), sodium tert-butoxide (1.9g, 20.0mmol) and (AMPHOS) were added to a 250mL two-necked flask 2 PdCl 2 (0.35g, 0.5mmol), add o-xylene 60mL, and react the system at 120...
PUM
| Property | Measurement | Unit |
|---|---|---|
| full width at half maximum | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com