Condensed ring compound containing two boron atoms and one or three chalcogen atoms and organic electroluminescent device
A technology of boron atoms and compounds, which is applied in the fields of compounds containing group 3/13 elements of the periodic table, electric solid-state devices, organic chemistry, etc., can solve problems such as complex device structures and reduced external quantum efficiency of devices, and reduce relaxation degree, narrow half-width, and high device external quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0093] The present invention does not have special limitation to the preparation method of described condensed ring compound, a typical preparation process of the compound shown in formula I is as follows:
[0094]
[0095] Among them, Ar' is Ar 2 and / or Ar 3 and / or Ar 4 , R' is R 2 and / or R 3 and / or R 4 , n' is n 2 and / or n 3 and / or n 4 .
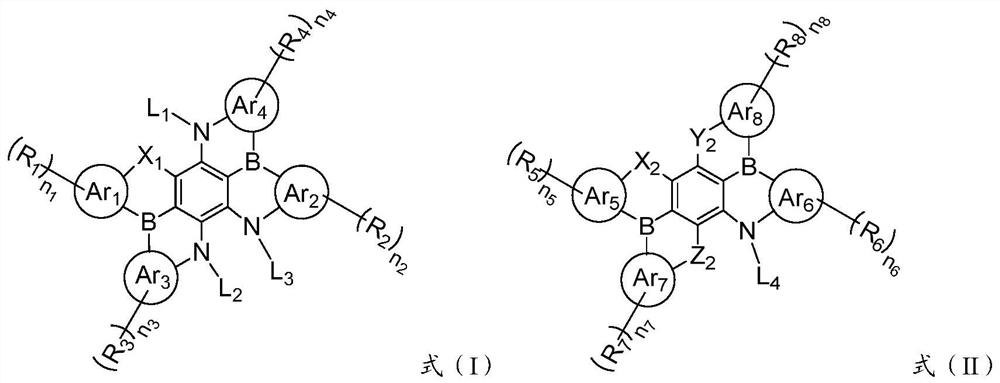
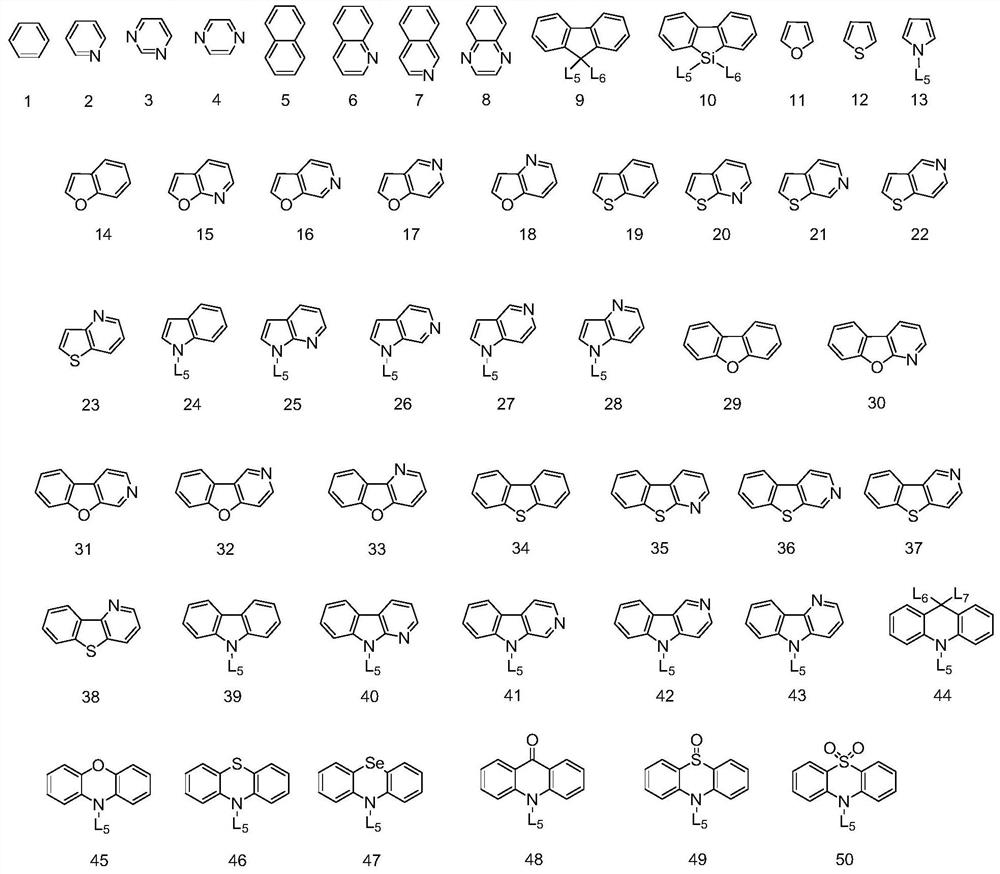
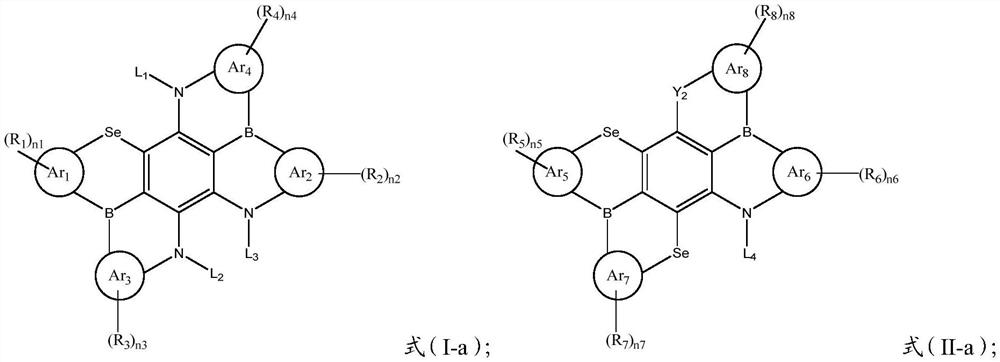
[0096] Formula Ar 1 ~ Ar 4 , R 1 ~R 4 , L 1 ~ L 4 , n 1 ~n 4 and X 1 They are all consistent with those described in the foregoing technical solutions, and will not be described here one by one.
[0097] A typical preparation process of the compound shown in formula II is as follows:
[0098]
[0099] in:
[0100] Formula Ar 5 ~ Ar 8 , R 5 ~R 8 , L 1 and n 1 ~n 4 、X 2 , Y 2 and Z 2 They are all consistent with those described in the foregoing technical solutions, and will not be described here one by one.
[0101] The present invention also provides an organic electroluminescent device, comprising an ano...
Embodiment 1
[0118] The reaction formula is as follows:
[0119]
[0120] Under argon atmosphere, 1-1(1,4-dibromo-2,3,5,6-tetrafluorobenzene) (10.0g, 0.032mol), diphenylamine (16.2g, 0.096 mol) and cesium carbonate (41.8g, 0.128mol), take 300mL N,N-dimethylformamide (DMF) into the bottle, raise the temperature to 130°C, stir and react for 3 hours, after cooling to room temperature, add the reaction solution to Settled in 500 mL of saturated brine, filtered, and dried in vacuo. The resulting solid was separated through a silica gel column to obtain product 1-2 (11.4 g, yield: 48%).
[0121] Elemental analysis structure (C 42 h 30 Br 2 FN 3 ): theoretical value C, 66.77; H, 4.00; Br, 21.15; F, 2.51; N, 5.56 test value C, 66.77; H, 4.01; N, 5.56.
[0122] Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) mass spectrometry: theoretical value 753.1; experimental value 753.1 (M + ).
[0123] Under argon atmosphere, 1-2 (11.5g, 15.22mmol), 4-methylbe...
Embodiment 2
[0131] The reaction formula is as follows:
[0132]
[0133] Under argon atmosphere, add 1-1 (14.4g, 0.047mol), 3,6-di-tert-butylcarbazole (39.4g, 0.14mol) and cesium carbonate (68.9g, 0.21mol) into a 500mL three-necked flask , put 200mL of N,N-dimethylformamide (DMF) into the bottle, heat up to 130°C, stir and react for 3 hours, after cooling to room temperature, add the reaction solution to 400mL of saturated saline for sedimentation, filter, and vacuum-dry to obtain The solid was separated through a silica gel column to obtain product 2-1 (23.9 g, yield: 47%).
[0134] Elemental analysis structure (C 66 h 72 Br 2 FN 3 ): theoretical value C, 72.99; H, 6.68; Br, 14.71; F, 1.75; N, 3.87 test value C, 72.99; H, 6.67; N, 3.87.
[0135] MALDI-TOF mass spectrum: theoretical value 1083.4; experimental value 1083.5 (M + ).
[0136] Under argon atmosphere, 2-1 (10.9g, 10.1mmol), 4-isopropylphenylselenol (2.02g, 10.1mmol) and sodium carbonate (1.07g, 10.1mmol) were added to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com