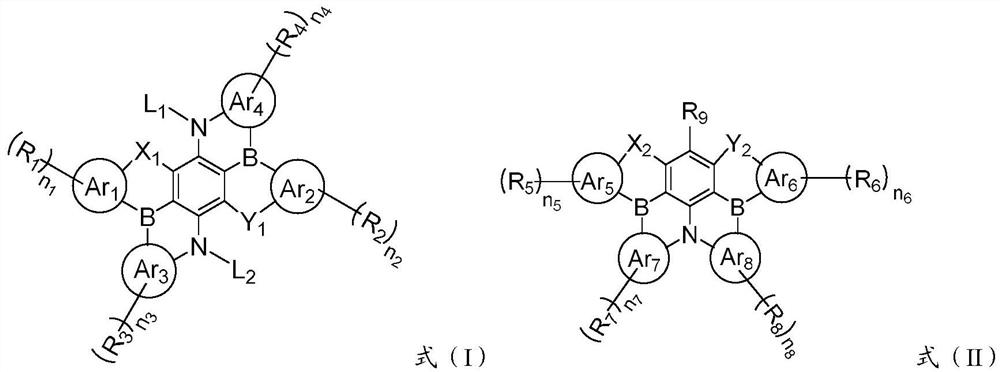
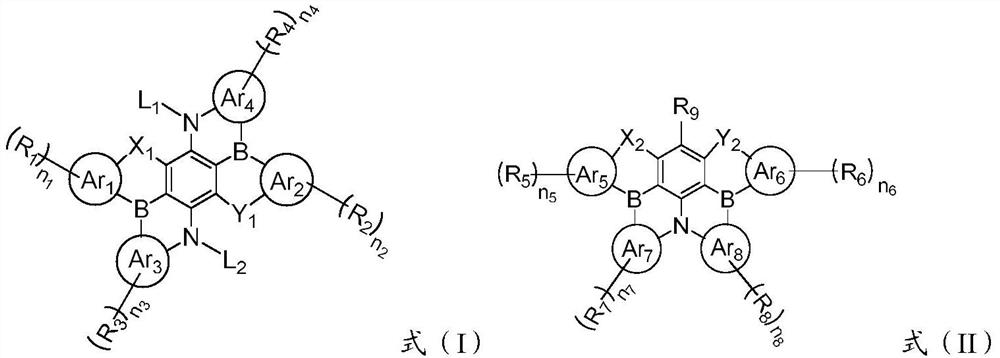
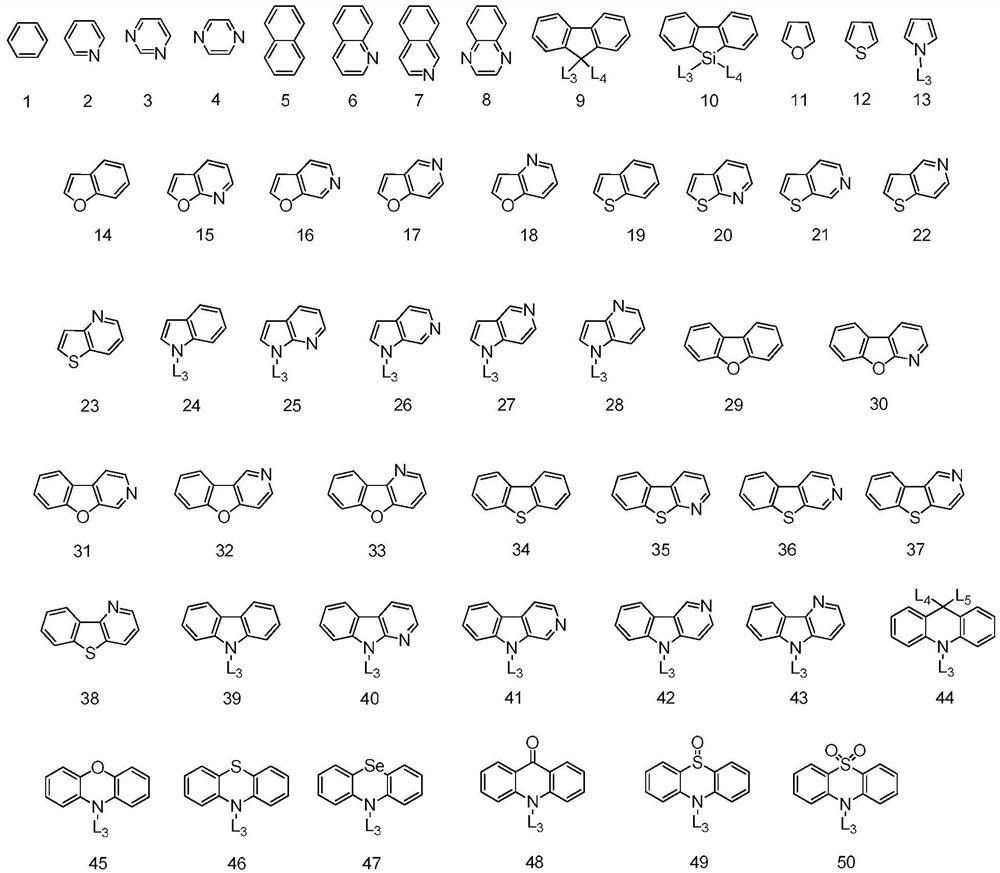
A condensed ring compound containing two boron atoms and two oxygen group atoms and an organic electroluminescent device
A compound and boron atom technology, applied in the field of organic light-emitting materials, can solve the problems of complex device structure and reduced external quantum efficiency of the device, and achieve the effects of reducing the degree of relaxation, narrowing the half-peak width, and narrowing the half-peak width of electroluminescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0106] The present invention also provides a method for preparing the above-mentioned fused ring compound containing two boron atoms and two oxygen atoms, comprising: combining the compound represented by formula (III) or the compound represented by formula (IV) with an alkyl group After the lithium reaction, react with boron trihalide and organic amine to obtain a fused ring compound represented by formula (I) or formula (II); the alkyl lithium is preferably butyllithium, sec-butyllithium, tert-butyllithium One or more of lithium, methyl lithium and ethyl lithium; the boron trihalide is preferably one or more of boron trifluoride, boron trichloride, boron tribromide and boron triiodide ; The organic amine is preferably one or more of N,N-diisopropylethylamine, triethylamine and tri-n-butylamine.
[0107]
[0108] Among them, Lu 1 ~Lu 4 It is hydrogen or halogen; other codes are the same as above, and will not be repeated here.
[0109] The present invention also provide...
Embodiment 1
[0124] The reaction formula is as follows:
[0125]
[0126] Under argon atmosphere, 1-1 (10.0g, 0.037mol), bis(4-tert-butylphenyl)amine (20.7g, 0.074mol), tris(dibenzylideneacetone) were added to a 500mL three-necked flask Dipalladium (1.7 g, 1.85 mmol), tri-tert-butylphosphorus tetrafluoroborate (2.1 g, 7.4 mmol) and sodium tert-butoxide (10.7 g, 0.11 mol) were added with 250 mL of toluene. The temperature was raised to 105°C, and the reaction was stirred for 3 hours. After cooling to room temperature, the reaction solution was extracted with 200 mL of ether, washed with saturated brine three times (200 mL×3), the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. Product 1-2 (19.4 g, yield: 78%).
[0127] Elemental Analysis Structure (C 46 H 54 F 2 N 2 ): Theoretical C, 82.10; H, 8.09; F, 5.65; N, 4.16 Tested C, 82.10; H, 8.09; N, 4.16.
[0128] Matrix-assisted laser desorption ionization ti...
Embodiment 2
[0141] The reaction formula is as follows:
[0142]
[0143] Under an argon atmosphere, 2-1 (30.0 g, 0.097 mol), 3,6-di-tert-butylcarbazole (53.0 g, 0.19 mol) and cesium carbonate (142.4 g, 0.44 mol) were added to a 500 mL three-necked flask , take 200 mL of N,N-dimethylformamide (DMF) into the bottle, heat up to 130 ° C, stir and react for 3 hours, after cooling to room temperature, add the reaction solution to 400 mL of saturated brine to settle, filter, and vacuum dry. The obtained The solid was separated by silica gel column to obtain product 2-2 (36.1 g, yield: 45%).
[0144] Elemental Analysis Structure (C 46 H 48 Br 2 F 2 N 2 ): Theoretical C, 66.83; H, 5.85; Br, 19,33; F, 4.60; N, 3.39 Tested C, 66.82; H, 5.89; N, 3.28.
[0145] MALDI-TOF mass spectrum: theoretical value 824.2; experimental value 824.3 (M + ).
[0146] Under argon atmosphere, add 2-2 (10.0g, 12.10mmol), thiophenol (3.4g, 25.40mmol) and sodium carbonate (2.7g, 25.40mmol) to a 500mL three-neck...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com