Liquid prothrombin time determination kit and preparation method thereof
A prothrombin time and kit technology, applied in biochemical equipment and methods, biological testing, microbial measurement/inspection, etc., can solve problems such as obvious differences between batches, complicated production process, and large cost investment, and achieve simplification Production process, simplify the operation process, solve the effect of inter-batch difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1.1 The liquid prothrombin time assay kit of the present invention comprises:
[0031] Thromboplastin is recombinant human tissue factor and rabbit brain powder, recombinant human tissue factor accounts for 0.05wt% of the weight of the buffer, and rabbit brain powder accounts for 5wt% of the weight of the buffer;
[0032] Stabilizers are trehalose, potassium sorbate and gentamicin sulfate, trehalose accounts for 2wt% of the buffer weight, potassium sorbate accounts for 1wt% of the buffer weight, and gentamicin sulfate accounts for 0.01 wt% of the buffer weight wt%;
[0033] Surfactant is polyethylene glycol 6000 and polyoxyethylene lauryl ether, polyethylene glycol 6000 accounts for 9wt% of buffer solution weight, polyoxyethylene lauryl alcohol accounts for 0.5wt% of buffer solution weight;
[0034] The salt ions are calcium chloride, sodium chloride and potassium sulfate, the concentration of calcium chloride is 15mM, the concentration of sodium chloride is 25mM, and ...
Embodiment 2
[0060] Embodiment 2 verifies the effect of kit of the present invention
[0061] 2.1 Repeatability test
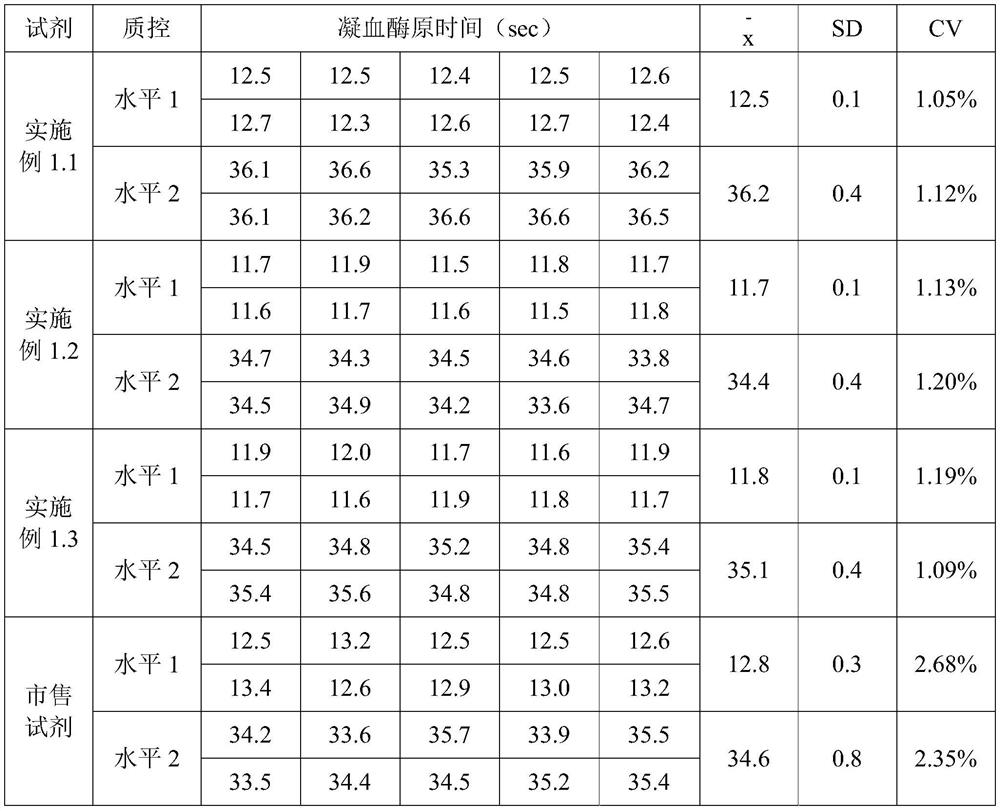
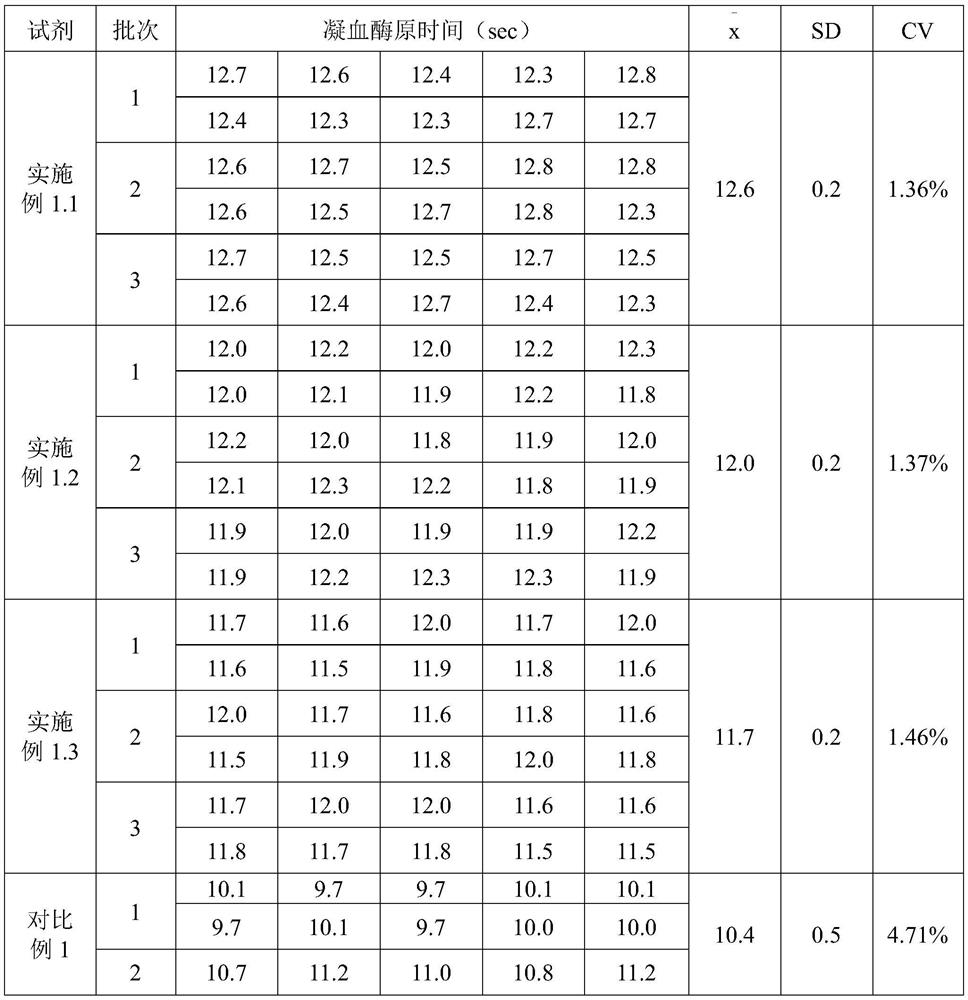
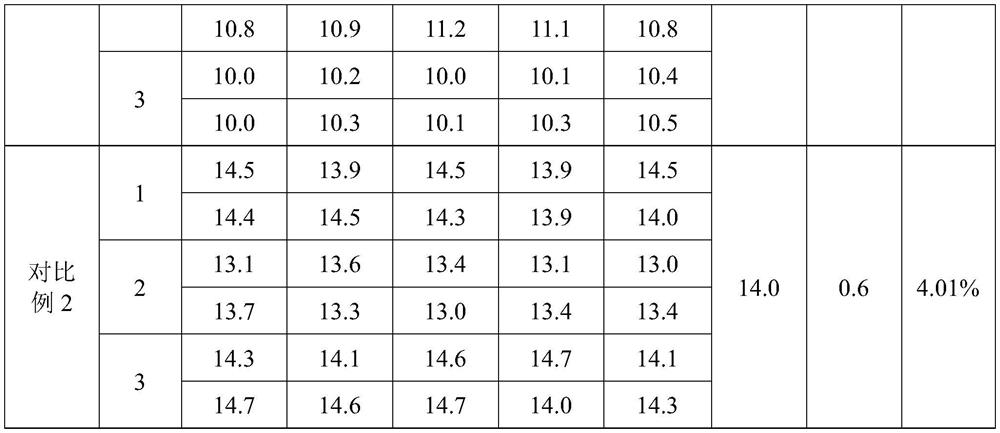
[0062] Carry out reagent preparation production according to 3 prescriptions in the embodiment of the present invention 1, the reagent produced and commercially available reagent test the blood coagulation of simens simultaneously on the CA1500 automatic coagulation instrument that Japanese SYSMEX (Hysmex) Co., Ltd. produces The prothrombin time (PT) of quality control plasma level 1 and level 2, the quality control of each level was repeatedly measured 10 times, and the average value of 10 measured values was calculated Standard deviation (SD) and coefficient of variation (CV), the measurement results are compared as shown in Table 1.
[0063] Table 1 Comparison table of repeatability test results
[0064]
[0065] As can be seen from Table 1, the coefficient of variation (CV) value of the repeatability test result of the kit of the present invention≤1.20%, less t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com