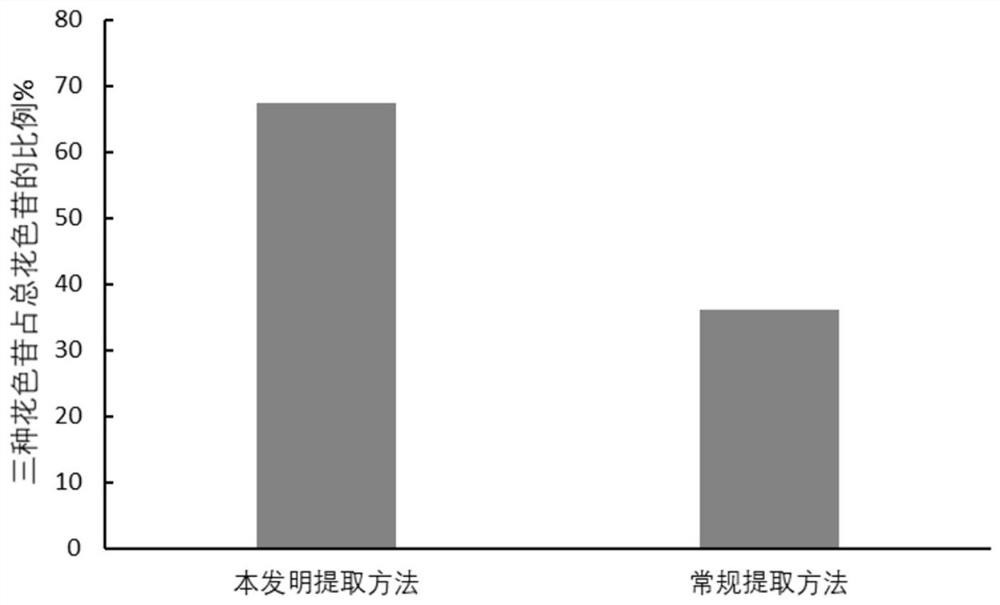
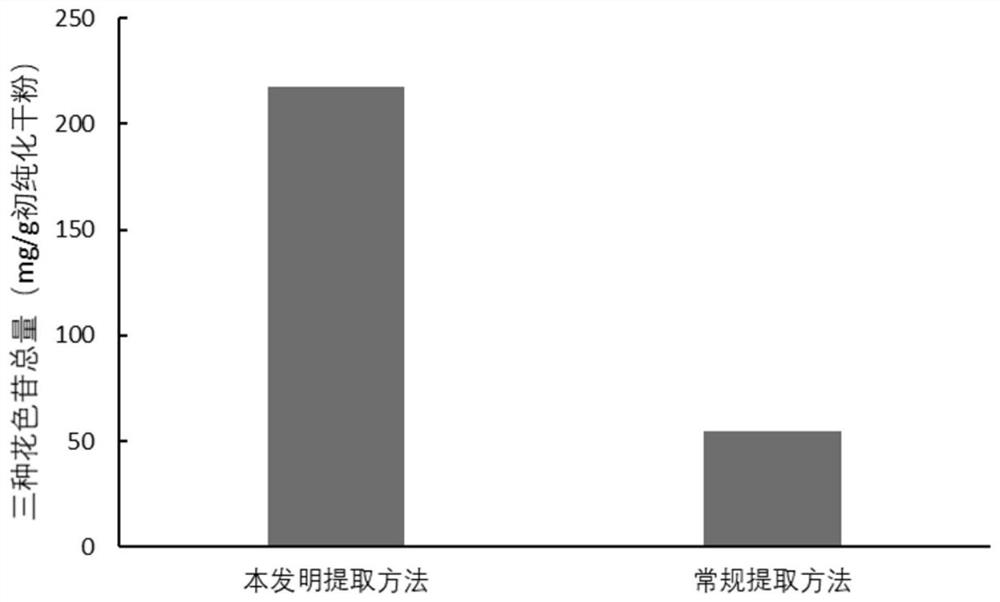
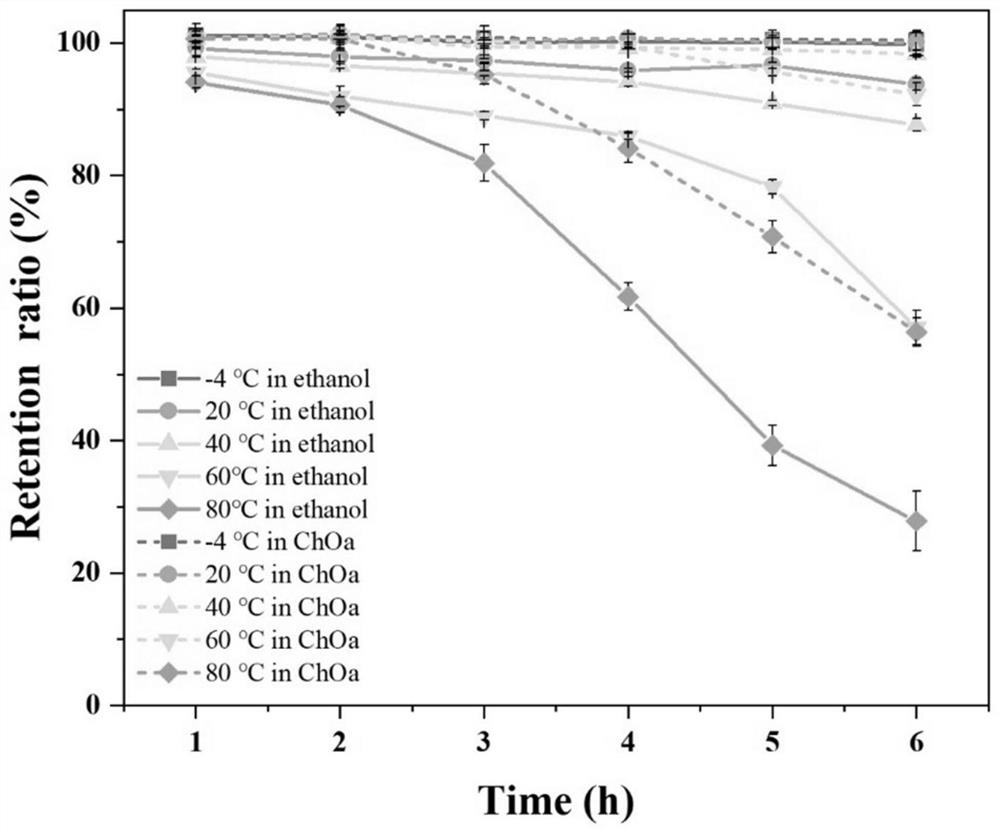
Application of natural eutectic solvent in selective extraction of anthocyanin and preservation of anthocyanin
A deep eutectic solvent and anthocyanin technology, which is applied in the field of food science and engineering, can solve the problems of poor stability of anthocyanins, affecting the physiological activity of color rendering, loss of anthocyanins, etc., to achieve protection stability and improve stability , the effect of inhibiting degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] This example provides a method for simultaneously extracting delphinidin-3-galactoside / arabinoside and mallow pigment-3-arabinoside with strong selectivity from blueberry pomace, while protecting its stability method, comprising the steps of:
[0036] Step (1): Freeze the freshly squeezed blueberry pomace, and pre-freeze at -20°C for 24 hours, then vacuum freeze-dry in a large freeze dryer for 36 hours, crush and grind, and avoid light during the whole process;
[0037] Step (2): Use choline chloride as the hydrogen bond acceptor and oxalic acid as the hydrogen bond donor. The molar ratio of the two is 2:1. It needs to be heated in a water bath at 80°C and kept stirring to avoid local denaturation and obtain clarification After transparent and homogeneous liquid (natural deep eutectic solvent), the amount of water added is 30% (w / w, the mass ratio of water to natural deep eutectic solvent), mixed with the sample powder in step (1), the liquid-solid ratio It is 50mL / g, ...
Embodiment 2
[0041]This example provides a method for simultaneously extracting delphinidin-3-galactoside / arabinoside and mallow pigment-3-arabinoside with strong selectivity from blueberry pomace, while protecting its stability The technical solution includes the following steps:
[0042] Step (1): Freeze the freshly squeezed pomace, and pre-freeze at -40°C for 24 hours, then vacuum freeze-dry in a large freeze dryer for 36 hours, crush and grind, and avoid light during the whole process;
[0043] Step (2): Use choline chloride as the hydrogen bond acceptor and oxalic acid as the hydrogen bond donor with a molar ratio of 1:1. It needs to be heated in a water bath at 80°C and kept stirring to avoid local denaturation and obtain a clear and transparent After the homogeneous liquid, add 30% (w / w) of water, mix with the sample powder in step (1), the liquid-solid ratio is 60mL / g, preheat at 45°C for 5min, make the sample powder in the natural eutectic Uniform diffusion in the solvent;
[00...
Embodiment 3
[0047] This example provides a method for simultaneously extracting delphinidin-3-galactoside / arabinoside and mallow pigment-3-arabinoside with strong selectivity from blueberry pomace, while protecting its stability The technical solution includes the following steps:
[0048] Step (1): Freeze the freshly squeezed pomace, and pre-freeze at -60°C for 24 hours, then vacuum freeze-dry in a large freeze dryer for 24 hours, crush and grind, and avoid light during the whole process;
[0049] Step (2): Use choline chloride as the hydrogen bond acceptor and oxalic acid as the hydrogen bond donor with a molar ratio of 1:2. It needs to be heated in a water bath at 80°C with constant stirring to avoid local denaturation and obtain a clear and transparent After a uniform liquid, add 30% (w / w) of water, mix with the sample powder in step (1), the liquid-solid ratio is 60mL / g, preheat at 40°C for 8min, and make the sample powder in the natural eutectic Uniform diffusion in the solvent;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com