In-situ gel for ceftiofur injection and preparation method thereof
A technology of ceftiofur and in-situ gel, which is applied in the directions of non-active ingredients medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc. Simple, slow-release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
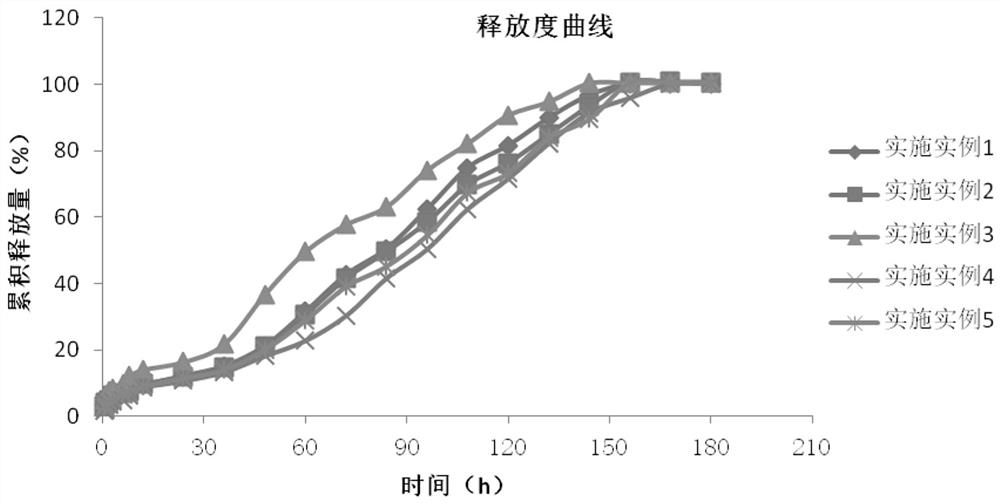
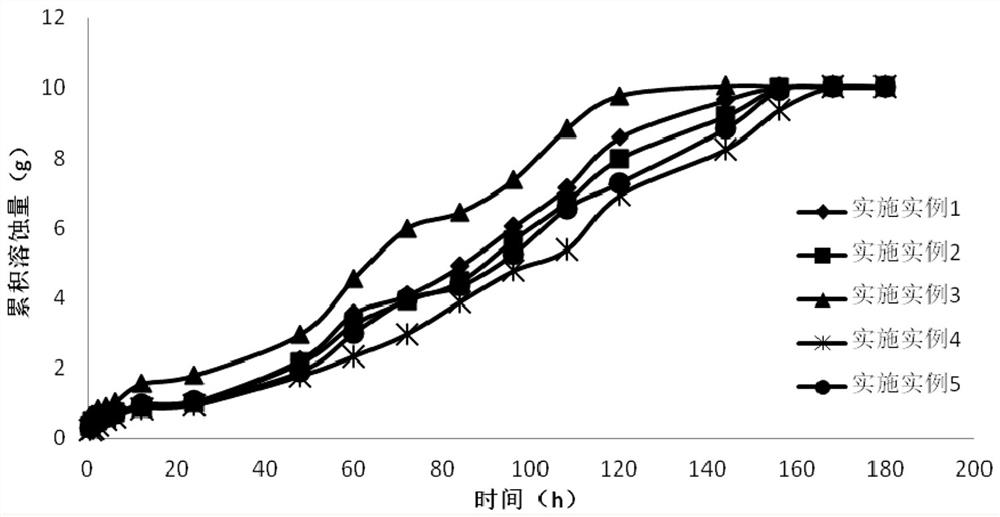
Examples
Embodiment 1
[0029] Ceftiofur solid dispersion prescription:
[0030]
[0031] The preparation of ceftiofur solid dispersion: (1) take stearic acid, soybean lecithin and cholesterol of prescription quantity and melt at 70 ℃, add the ceftiofur of prescription quantity, stir evenly; (2) put in -20 ℃ quickly (3) low-temperature pulverization to obtain ceftiofur solid dispersion.
[0032] In situ gel preparation prescription for injection:
[0033]
[0034]
[0035] The preparation method of in situ gel for ceftiofur injection:
[0036] Step 1: Take the prescribed amount of ceftiofur solid dispersion, methylcellulose and poloxamer 188 and mix evenly, then add 80% of the prescribed amount to water, and stir until the poloxamer 188 is completely dissolved.
[0037] Step 2: Take the prescribed amount of poloxamer 407 and add it to step 1, stir until a lump-free and uniformly dispersed medicinal solution is obtained, add water to make up the volume, and the product is ready.
[0038] I...
Embodiment 2
[0047] Ceftiofur solid dispersion prescription:
[0048]
[0049] The preparation of ceftiofur solid dispersion: (1) take glycerol monostearate, hydrogenated soybean lecithin and cholesterol of prescription quantity and melt at 85 ℃, add the ceftiofur of prescription quantity, stir evenly; (2) place rapidly Cooling and solidification at -20°C; (3) Low-temperature pulverization to obtain a solid dispersion of ceftiofur.
[0050] In situ gel preparation prescription for injection:
[0051]
[0052] Preparation:
[0053] The preparation method of in situ gel for ceftiofur injection:
[0054] Step 1: Take the prescribed amount of ceftiofur solid dispersion, povidone K30 and poloxamer 188, mix evenly, add 80% of the prescribed amount to water, stir until the poloxamer 188 is completely dissolved, and the ceftiofur solid is dispersed The body is evenly dispersed.
[0055] Step 2: Take the prescribed amount of poloxamer 407 and add it to step 1, stir until a lump-free and u...
Embodiment 3
[0058] Ceftiofur solid dispersion prescription:
[0059] Ceftiofur 200.0g
[0060] Glyceryl Palmitate 350.0g
[0061] Soy Sterol 100.0g
[0062] Preparation:
[0063] Preparation of ceftiofur solid dispersion: (1) Take glyceryl palmitate and soy sterol of the prescription amount and melt at 80°C, add the ceftiofur in the prescription amount, and stir evenly; (2) rapidly place at -20°C Cooling and solidification; (3) low-temperature pulverization to obtain a solid dispersion of ceftiofur.
[0064] In situ gel preparation prescription for injection:
[0065]
[0066] The preparation method of in situ gel for ceftiofur injection:
[0067] Step 1: Take the prescribed amount of ceftiofur solid dispersion, hydroxypropyl methylcellulose and poloxamer 188 and mix evenly, add 80% of the prescribed amount to water, stir until the poloxamer 188 is completely dissolved, and the cefotaxime The furan solid dispersion is uniformly dispersed.
[0068] Step 2: Take the prescribed amo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com