Frozen microneedle array, preparation method therefor and application of frozen microneedle array
A microneedle array and microneedle technology, applied in the field of biomedical materials, can solve the problems of easy inactivation of biologically active substances, limited range of material selection, and high requirements for the preparation process, and achieve low technical requirements, universality, and simple methods. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
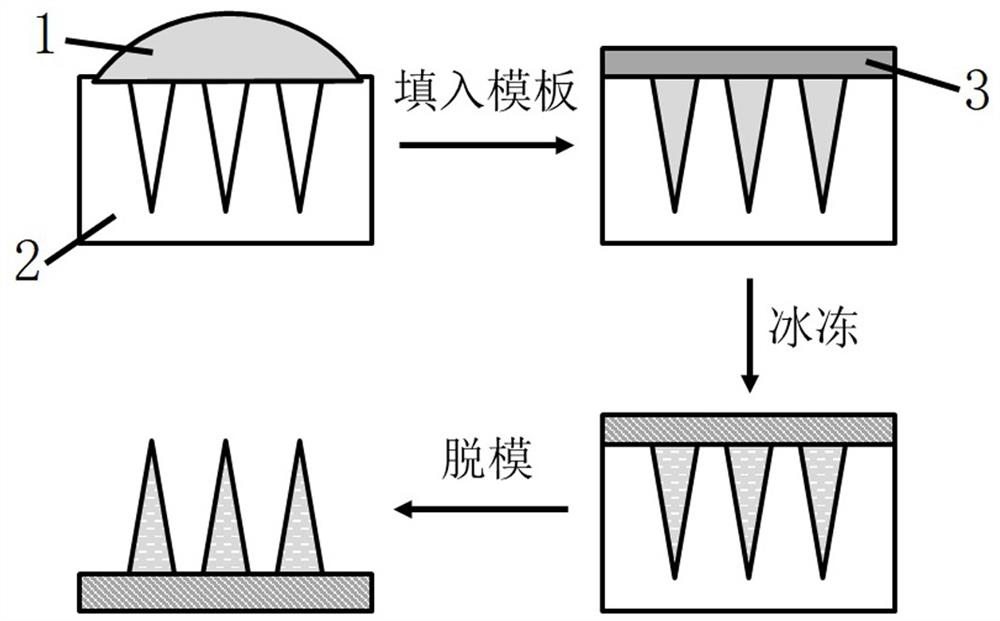
[0040] A kind of frozen methacrylate gelatin microneedle carrying rhodamine B, its preparation comprises the following steps:
[0041] S1. Prepare drug-loaded microneedle raw material solution A: prepare an aqueous solution containing 15% methacrylate gelatin by mass fraction and 1% 2-hydroxy-2-methylpropiophenone by volume, and mix 1 mg / mL of Rhodamine B;
[0042] S2. Preparation of drug-loaded frozen microneedle array
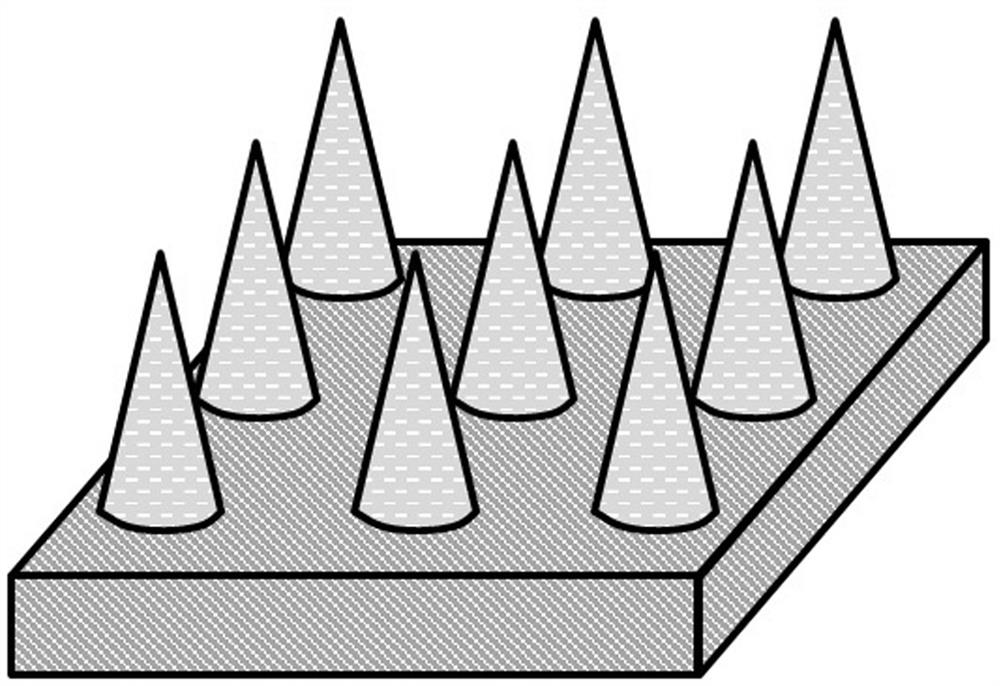
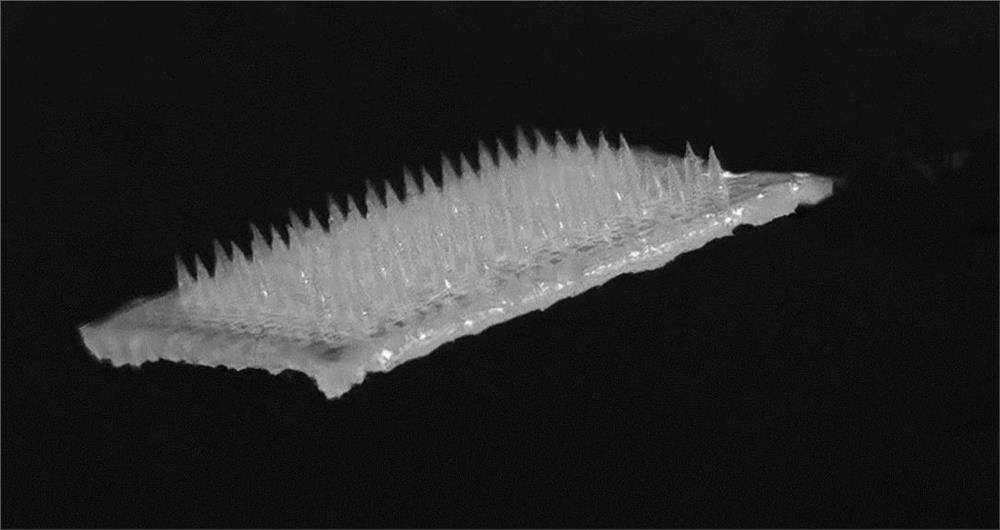
[0043] S21. Use a pipette gun to drop 100 μl of the drug-loaded microneedle raw material solution A onto the polydimethylsiloxane template 2 with an array of inverted conical holes (the hole spacing is 200 μm, the hole depth is 300 μm, and the hole diameter is 150 μm). Surface, vacuum treatment for 5 minutes to make it fully filled in the inverted conical hole, use a pipette gun to suck away the excess microneedle raw material solution 1 outside the inverted conical hole, and irradiate with ultraviolet light for 20s to make the drug-loaded drug in the inver...
Embodiment 2
[0047] A frozen calcium alginate microneedle carrying green fluorescently labeled insulin, the preparation of which comprises the following steps:
[0048] S1. Prepare drug-loaded microneedle raw material solution B: prepare a very low-viscosity sodium alginate solution with a mass fraction of 4%, and mix green fluorescently labeled insulin (final content 0.5 mg / mL) into it;
[0049] S2. Preparation of drug-loaded frozen microneedle array
[0050] S21. Use a pipette gun to drop 400 μl of the drug-loaded microneedle raw material solution B onto the polydimethylsiloxane template 2 with an array of inverted conical holes (the hole spacing is 700 μm, the hole depth is 850 μm, and the hole diameter is 600 μm). Surface, centrifuged at 1000rpm for 3min to make it fully filled in the inverted conical hole, use a pipette gun to suck away the excess drug-loaded microneedle material solution B outside the inverted conical hole, and drop 400μl mass fraction of 10 to the surface of the hol...
Embodiment 3
[0054] A kind of frozen Matrigel microneedle carrying Bacillus subtilis, its preparation comprises the steps:
[0055] S1. Prepare the microneedle raw material solution C carrying living microorganisms: mix Bacillus subtilis (purchased from Beina Biological Co., Ltd.) with Matrigel (Corning, USA), and the final content of Bacillus subtilis is 10 8 / mL;
[0056] S2. Preparation of drug-loaded frozen microneedle array
[0057] S21. Use a pipette gun to drop 300 μl of the drug-loaded microneedle raw material solution C onto the polydimethylsiloxane template with an array of inverted pyramidal holes (the hole spacing is 400 μm, the hole depth is 800 μm, and the hole diameter is 600 μm). 2 surface, centrifuge at 1000rpm for 3min to make it fully filled in the inverted pyramid-shaped hole, use a pipette gun to suck away the excess drug-loaded microneedle material solution C outside the inverted conical hole, and place it at 37°C for 20min , the drug-loaded microneedle raw material...
PUM
| Property | Measurement | Unit |
|---|---|---|
| depth | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com